This decision memorandum does not constitute a national coverage determination (NCD). It states the intent of the Centers for Medicare & Medicaid Services (CMS) to issue an NCD. Prior to any new or modified policy taking effect, CMS must first issue a manual instruction, program memorandum, CMS ruling or Federal Register Notice, giving specific directions to our claims processing contractors. That issuance, which includes an effective date, is the NCD. If appropriate, the Agency must also change billing and claims processing systems and issue related instructions to allow for payment. The NCD will be published in the Medicare Coverage Issues Manual. Policy changes become effective as of the date listed in the transmittal that announces the Coverage Issues Manual revision.
To: Administrative File: CAG 00066R3
Ocular Photodynamic Therapy with Verteporfin for Macular Degeneration
From: Steve E. Phurrough, MD, MPA
Director, Coverage and Analysis Group
Jesse Polansky, MD
Director, Division of Items and Devices
Stuart Caplan RN, MAS
Lead Analyst
Marc Stone, MD
Lead Medical Officer
Carlos Cano, MD
Medical Officer
Subject: Coverage Decision Memorandum for Ocular Photodynamic Therapy with Verteporfin for Macular Degeneration
Date: January 28, 2004
I. Decision
CMS has determined that the evidence is adequate to conclude that ocular photodynamic therapy (OPT) with verteporfin is reasonable and necessary for treating (1) subfoveal occult with no classic choroidal neovascularization (CNV) associated with age-related macular degeneration (AMD) and (2) subfoveal minimally classic choroidal neovascularization associated with age-related macular degeneration. These indications for ocular photodynamic therapy with verteporfin are reasonable and necessary for both (1) and (2) only when:
- The lesions are small (4 disk areas or less in size) at the time of initial treatment or within the three months prior to initial treatment; and
- The lesions have shown evidence of progression within the three months prior to initial treatment. Evidence of progression must be documented by deterioration of visual acuity (at least five letters on a standard examination), lesion growth (an increase in one disk area), or the new appearance of blood in the lesion.
Therefore we intend to issue a national coverage determination (NCD) expanding coverage of OPT with verteporfin for subfoveal occult with no classic CNV associated with AMD and for subfoveal minimally classic CNV associated with AMD for the stated indications.
This decision memorandum does not alter our national coverage policy for OPT with verteporfin for subfoveal predominantly classic CNV associated with AMD.
Other uses of OPT with verteporfin to treat AMD not already addressed by CMS will continue to be non-covered. These include but are not limited to the following AMD indications:
- Patients with juxtafoveal or extrafoveal CNV lesions (lesions outside the fovea)
- Patients who are unable to obtain a fluorescein angiogram
- Patients with atrophic or "dry" AMD.
OPT with verteporfin for other ocular indications, such as pathologic myopia or the presumed ocular histoplasmosis syndrome, is not addressed in this decision memorandum nor in our national coverage policy, and continues to be eligible for coverage through individual contractor discretion.
II. Background
Age-related macular degeneration is the leading cause of legal blindness in Americans over the age of 65. The estimated prevalence of AMD in Americans 75 years of age or older is 7.1%.1 While the exact etiology of AMD is not well understood, it is thought to be a multi-factorial disease. In addition to age, several other risk factors are associated with AMD. These include family history of AMD, smoking, and light eye color. Recent findings also suggest that low dietary intake of antioxidants may predispose people to AMD.
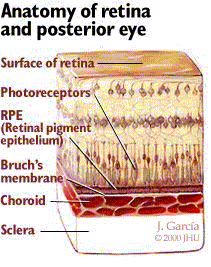
AMD involves the destruction of normal macular function. In AMD, acellular debris called drusen accumulates within Bruch's membrane. Bruch's membrane, as shown in Figure 1, is the layer between the outer edge of the retina and the choroid. This key layer keeps the blood vessels of the choroid from leaking fluid into the retina.
There are two basic types of AMD: dry and wet. Dry AMD is the most common type, accounting for 90% of all cases. In dry AMD, the accumulation of drusen, and the resulting effect it has on macular function, leads to central vision deterioration. While AMD is the most common condition associated with CNV, other retinal disorders such as pathologic myopia, presumed ocular histoplasmosis syndrome, angioid streaks, and retinal hamartomas can be complicated by CNV formation. Wet AMD accounts for 10% of cases and poses a higher risk of severe central vision loss. In wet AMD, breaks in Bruch's membrane allow vessels from the choroid to grow, leak, and bleed into the subretinal space; this is termed choroidal neovascularization (CNV). CNV can cause large distortions of the macula and can progress quickly (over the course of days or weeks), effectively destroying central vision. There is no definitive treatment for dry AMD. For patients with wet AMD, laser photocoagulation has been shown to help reduce the rate of vision loss in some patients.
Patients suspected of having wet AMD generally undergo fluorescein angiography. There are two basic patterns of fluorescein leakage in wet AMD: classic and occult. In pure classic CNV, the choriocapillaris plexuses that are involved can be seen distinctly. In pure occult lesions, the location of the offending vessels responsible for the leakage is not recognizable. Many CNV lesions are a combination of both occult and classic with a portion showing a defined site of leakage and another portion being obscured. CNV in AMD is further characterized by one of three locations: subfoveal, juxtafoveal and extrafoveal. Subfoveal, as the name implies, is CNV that lies directly below the fovea. Juxtafoveal and extrafoveal CNV lie progressively further away from the fovea (but still within the macula).
Laser photocoagulation has been shown to decrease vision loss by 50% in juxtafoveal and extrafoveal CNV. For subfoveal CNV, laser treatment has been shown to have some benefit, mainly in patients with classic CNV. Laser photocoagulation by itself destroys the retina overlying the area of application. When applied away from the foveal center (i.e., juxtafoveal or extrafoveal) the effect of the laser itself on vision is variable. When applied to the foveal center, as in cases of subfoveal CNV, the laser is almost assured to destroy some central vision. In addition, subfoveal CNV recurs approximately 50% of the time after "successful" laser therapy. Thus, while laser photocoagulation of subfoveal CNV theoretically may be preferable to allowing the disease to progress naturally, that possible benefit carries potential risks.
Ocular Photodynamic Therapy with Verteporfin: OPT for the treatment of CNV involves the intravenous injection of a photosensitive drug, verteporfin. A laser, which emits light only at verteporfin's absorption peak of 689 nm, is then directed into the eye. It is thought that the excitation of verteporfin generates singlet oxygen and other reactive intermediates that result in temporary closure of leaking blood vessels. The laser is non-thermal; thus it does not produce a heat effect on the retina and causes no damage to the retinal tissue. Verteporfin therapy is neither a cure nor a preventative for CNV in AMD; it is meant to slow progression of the disease. Indeed, its effect is generally not permanent. The closure of leaking blood vessels caused by OPT is often temporary, and these vessels may re-open. Additional OPT treatments, therefore, may be needed.
III. History of Medicare Coverage
On November 8, 2000, the Centers for Medicare and Medicaid Services (CMS), then the Health Care Financing Administration, announced its decision to cover OPT with verteporfin for AMD patients with predominantly classic CNV lesions (where the area of classic CNV occupies more than 50% of the area of the entire lesion) as determined by a fluorescein angiogram. CNV lesions are made up of classic and/or occult components. This policy became effective on July 1, 2001.
Subsequently, CMS became aware of evidence not previously considered addressing the therapy's effectiveness in AMD patients with subfoveal occult CNV with no classic CNV (i.e. patients with 0% classic CNV). CMS reviewed the new evidence to determine if Medicare's national coverage decision on OPT with verteporfin should include this indication.
On October 17, 2001 CMS announced its intent to cover OPT with verteporfin for AMD patients with occult and no classic subfoveal CNV as determined by a fluorescein angiogram. After posting this Decision Memorandum, however, CMS discovered new issues concerning the data from the clinical trial upon which the agency had based its analysis.
On October 29, 2001, in order to further examine the clinical trial data, CMS internally generated a request for reconsideration of this indication. CMS did not implement the October 17, 2001 memorandum pending completion of the reconsideration.
On March 28, 2002, CMS announced its intent to reaffirm the current national noncoverage policy for OPT with verteporfin for AMD patients with occult and no classic subfoveal CNV as determined by a fluorescein angiogram.
IV. Timeline of Recent Activities
| DATE |
ACTIVITY |
| May 27, 2003 |
As part of an agreement to settle pending litigation, CMS agreed to convene a Medicare Coverage Advisory Committee (MCAC) meeting to evaluate scientific evidence and make non-binding recommendations to CMS regarding verteporfin for AMD patients with occult and no classic subfoveal CNV. |
| July 25, 2003 |
CMS opened a reconsideration of the current national noncoverage policy for OPT with verteporfin for AMD patients with occult and no classic CNV as determined by a fluorescein angiogram. |
| September 9, 2003 |
The MCAC convened to review the issue of OPT with verteporfin for AMD. |
| October 23, 2003 |
CMS posted the minutes and transcript of the September 9, 2003 MCAC meeting on the Medicare coverage website. |
V. FDA Status
On April 12, 2000, the Food and Drug Administration (FDA) approved the use of verteporfin in AMD-related subfoveal CNV in which more than 50% of the lesion is classic (i.e. predominantly classic) as determined on fluorescein angiogram. On August 22, 2001, the FDA approved verteporfin for the treatment of predominantly classic subfoveal CNV related to pathologic myopia as well as ocular histoplasmosis. The use of OPT with verteporfin for subfoveal occult with no classic CNV in AMD is an off-label use.
VI. General Methodological Principles of Study Design
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve net health outcomes for patients. The General Methodological Principles of Study Design is located in Appendix A.
Clinical Trial Design Issues Specifically Related to the Verteporfin Studies
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) has written guidelines for the design of clinical trials.2 The principal regulatory bodies in the United States, European Union, and Japan have accepted these guidelines as standards for the evaluation of evidence.
The ICH guidelines emphasize the need for a thorough specification of the analysis before the trial begins:
- “For each clinical trial … all important details of its design and conduct and the principal features of its proposed statistical analysis should be clearly specified in a protocol written before the trial begins.” (p.2)
- “…The trial’s primary objective is always pre-defined, and is the hypothesis that is subsequently tested when the trial is complete.” (p.4)
- “The primary analysis of the primary variable should be clearly distinguished from supporting analyses of the primary or secondary variables.” (p.26)
- “Dichotomisation or other categorisation of continuous or ordinal variables may sometimes be desirable…. The criteria for categorisation should be pre-defined and specified in the protocol, as knowledge of trial results could easily bias the choice of such criteria.” (pp.7-8)
VII. Evidence
A. Introduction
Consistent findings across studies of net health outcomes associated with an intervention as well as the magnitude of its risks and benefits are key to the coverage decision process. For this decision memorandum, CMS reviewed the clinical evidence on OPT with verteporfin to determine whether OPT with verteporfin in comparison with placebo improves the net health outcomes of patients with AMD-related occult CNV with no classic lesions. CMS reviewed the published studies reporting on the largest randomized clinical trials to date on the subject, which are known as the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study (TAP), and the Verteporfin In Photodynamic Therapy Study (VIP). In addition, our staff reviewed original and amended study protocols for these trials and unpublished additional analyses of study data. CMS also performed a search for any recent systematic review on OPT that summarized and critically appraised the literature on this topic.
Outcomes of interest were the beneficial and adverse clinical effects of ocular photodynamic therapy with verteporfin. Specifically, the beneficial effects of therapy should lead to a positive impact on the course of AMD. Without treatment, AMD, particularly “wet” AMD, generally leads to a continuing deterioration of vision. A positive effect of treatment should be mediated through improvement in vision or a slowing of the rate of visual loss. If such a positive effect on visual acuity exists, its benefit must be measured in terms of importance to the patient: superior function and quality of life. The impact of visual loss on patient function and quality of life depends greatly on whether the eye affected by treatment is the better-seeing eye. Adverse clinical effects may be due to negative effects of the treatment on vision but also include other forms of toxicity and injury as well as any other factors that may limit the treatment’s acceptability to patients.
Clinical investigators have utilized a variety of validated instruments and tests to measure these outcomes. The measurement of visual function is usually performed through the reading of letters on a standard chart. Both visual acuity and contrast sensitivity can be measured using different types of charts. Health related quality of life is measured through validated questionnaires. The National Eye Institute’s Visual Functioning Questionnaire-25 is among the best-recognized instruments of this type. Adverse effects may be recognized through conventional methods of patient complaints, history, physical examination, and laboratory testing. Adverse effects having an impact on vision may be reflected in visual function testing and in instruments that measure vision-related quality of life.
B. Discussion of evidence reviewed
1. Assessment questions
The development of an assessment in support of Medicare coverage decisions is based on the same general question for almost all requests: “Is the evidence sufficient to conclude that the application of the technology under study will improve health outcomes for Medicare patients?” The formulation of specific questions for the assessment recognizes that the effect of an intervention can depend substantially on how it is delivered, to whom it is applied, the alternatives with which it is being compared, and the delivery setting. In order to appraise the net health outcomes of ocular photodynamic therapy with verteporfin in comparison with placebo and identify any relevant patient and facility selection criteria, CMS sought to address the following questions:
- Is there adequate evidence to draw conclusions about the net health benefits, that is, whether or not the risks and benefits of treatment outweigh the risks and benefits of non-treatment of OPT with verteporfin in routine clinical use in the population of Medicare beneficiaries who have AMD and occult with no classic CNV?
- If there is adequate evidence, does the evidence demonstrate that OPT with verteporfin treatment improves net health outcomes in treating AMD in occult with no classic CNV?
2. External systematic reviews/technology assessments
Systematic reviews are based on a comprehensive and unbiased search of published studies to answer a clearly defined and specific clinical question such as that related to the clinical benefit of OPT with verteporfin. A well-defined strategy or protocol (established before the results of the individual studies are known) guides this literature search. Thus, the process of identifying studies for potential inclusion and the sources for finding such articles is explicitly documented at the start of the review. Finally, systematic reviews provide a detailed assessment of the studies included. 3
One systematic review on OPT through September 2001 that met these criteria was available to CMS during the literature evaluation. In this section, we summarize the findings of this technology assessment (TA) published by the British National Coordinating Centre for Health Technology Assessment (NCCHTA), which includes a systematic review of the clinical literature on the use of verteporfin in the management of CNV associated with AMD.
NCCHTA TA on Clinical Effectiveness and Cost Utility of OPT for Wet AMD. 4
The purpose of this assessment was to “establish the clinical and cost-effectiveness of photodynamic therapy for the neovascular form of wet AMD relative to current practice and in relation to current licensed indications.” The TA included a systematic review and economic evaluation of randomized controlled trials of verteporfin photodynamic therapy for wet AMD. The review included both the TAP and VIP trials.
Results
The authors considered the results of the TAP trial to be strongly positive and calculated that the treatment effect observed in the VIP trial was not statistically different from that of the TAP trial although the power to detect a difference between the two trials was limited. They also noted “extensive subgroup analyses were presented for both trials.” Particularly because the authors were unable to obtain access to the study protocols, they believe the subgroup results “should be treated with extreme caution and at best should be regarded as generating hypotheses requiring more research.” They observed that any preservation of visual acuity achieved with OPT with verteporfin cannot be easily translated into improvements in patient function and quality of life because of the likelihood that the eye requiring treatment was the eye with poorer acuity.
Appraisal
This assessment considered OPT with verteporfin to have efficacy in the treatment of “wet” AMD. The authors believed that the evidence was insufficient to determine whether the treatment was more or less efficacious in particular subgroups. They also had insufficient information to assess the treatment’s effect on patient function and quality of life. Because the assessment was concerned with cost-effectiveness, the lack of this additional information prevented any conclusions as to when this treatment should be given.
The TA concluded that there is a need for additional large, multicenter, well-designed RCTs. The authors believed that these trials should include parallel economic evaluations that directly measure quality of life and survival. They also stated that there was no data on the relationship between cost and benefits when AMD-related CNV affects the worse-seeing eye first.
3. Internal technology assessment
CMS staff performed a search for any additional OPT with verteporfin prospective comparative individual effectiveness studies that may have been published since the TAP and VIP studies. We found no additional studies meeting these criteria.
The scientific evidence considering verteporfin OPT use for patients with CNV due to AMD is concentrated on two studies: the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study (TAP) and the Verteporfin In Photodynamic Therapy Study (VIP). Below is a detailed summary of the findings of the two TAP and VIP studies. For this review, CMS staff had access to the published papers, original and amended study protocols, and unpublished additional analyses of study data. TAP preceded VIP, and the two studies had similar designs and outcome measures.
a) Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study (TAP.)5 6
The TAP investigation comprised two multi-center, randomized, placebo-controlled clinical trials that were specifically conducted “to determine if photodynamic therapy with verteporfin…can safely reduce the risk of vision loss in patients with subfoveal CNV caused by AMD.” The study enrolled 609 subjects in 22 ophthalmology practices in North America and Europe. This was the first major study of this drug with the primary objective of proving safety and efficacy sufficiently to allow the drug to be licensed.
Study design and methods
The primary efficacy outcome of the TAP study was loss of visual acuity, calculated as the proportion of patients (or eyes, only one eye was treated per patient) that had fewer than 15 letters lost (approximately three lines of visual acuity lost) compared to baseline at 12 months. A loss of three lines or more is indicative of moderate visual loss. Secondary efficacy outcomes included:
- Proportion of eyes that had fewer than 30 letters lost (approximately six lines of visual acuity loss) compared to baseline—a measure of severe visual acuity loss.
- Mean changes in numbers of letters read on a visual acuity chart.
- Mean changes in numbers of letters read on a contrast sensitivity chart.
- Angiographic outcomes (lesion size, leakage, and progression).
These secondary efficacy outcome measures were included to validate any observed changes in the primary efficacy outcome within and between the two treatment groups.
Important inclusion criteria were:
- CNV must be under the geometric center of the foveal avascular zone (subfoveal).
- Patients must have evidence of some classic CNV (with or without occult component) as determined by fluorescein angiography.
- Patients must have best-corrected visual acuity of between 73 and 34 letters (approximate Snellen equivalent of 20/40 through 20/200).
- Patients must be ≥ 50 years of age.
In both the TAP and VIP studies, patients were randomized to either the verteporfin treatment group or the placebo group at a ratio of 2:1 in favor of active treatment. Patients in the verteporfin group received an intravenous injection of 6 mg/m2 body surface area of verteporfin in 30 cc of 5% Dextrose. The placebo group received 30 cc of 5% Dextrose intravenously. After the infusion, the enrolled eye in both patient groups was exposed to a 689 nm wavelength nonthermal laser for 83 seconds.
All patients were scheduled for regular three-month follow-up visits. At each regularly scheduled exam, the patients’ vision was tested, a dilated fundus examination was performed, and a fluorescein angiogram done. Physicians assessing the patients at each follow-up, as well as patients, were blinded to the treatment group. If there was any leakage seen on the fluorescein angiogram, the patient was retreated with the same agent to which they were randomized. Patients were followed for 24 months.
Statistical considerations
To account for missing data points a “last observation carried forward” approach was used in the data analysis. To preserve randomization, guard against patient selection bias, and account for crossovers in the study groups, the protocols specified an “intent-to-treat” analysis as the primary analysis. The treatment protocols were maintained throughout the two-year time periods.
Results
Aggregate results of the 12 and 24-month examinations for the entire study population are given in Table 1. At 12 months of follow-up (TAP report 1), the verteporfin group had an overall lower risk of visual acuity loss compared with placebo. In addition, mean contrast sensitivity was better in the verteporfin group compared with placebo. Differences in angiographic outcomes between the two groups at 12 months reinforced the observed treatment benefit of verteporfin. The verteporfin group showed greater reductions in lesion size, leakage, and progression than the placebo group.
Table 1: TAP Study: Analysis of overall treatment effect
| Outcome |
Treatment Group |
12-Month Endpoint |
24-Month Endpoint |
|---|
| Loss of < 15 letters
% of eyes |
V |
61.2% |
53.0% |
| P |
46.4% |
38.0% |
| |
p<.001 |
p<.001 |
| Loss of ≥ 30 letters
% of eyes |
V |
14.7% |
18.2% |
P |
23.7% |
30.0% |
| |
p<.001 |
p<.001 |
V = verteporfin, P = placebo
The results of the 24-month examination (TAP report 2) corroborated those of the 12-month examination. At two years of follow-up, visual acuity and mean contrast sensitivity remained significantly better for the verteporfin group compared with placebo. The angiographic outcomes at the 24-month follow-up also continued to show improvements in CNV lesion size, leakage, and progression in the verteporfin group.
Despite the significant improvements in angiographic outcome measures and the slowed rate of visual acuity loss observed in the verteporfin group, some leakage from the treated subfoveal CNV lesion still occurred. Additional treatments were only administered to study participants if fluorescein angiography, which was performed every three months, revealed CNV leakage. If no leakage was detected, patients were not retreated. For the verteporfin group, patients were retreated an average of 3.4 times per participant during the first year of the study and 2.2 times during the second year of the study for a total of 5.6 treatments per participant over a two year period. In comparison, the placebo group was retreated an average of 3.7 times during the first year and 2.8 times during the second year for a total of 6.5 treatment per participant over two years.
Neither TAP report 1 nor 2 addressed the issue of treatment cessation, an important concern given that an average patient received nearly six treatments over a two-year period. The appropriate frequency of treatment, the criteria needed to determine treatment failure, and the appropriate number of treatments needed beyond two years are questions that remain unanswered.
Subgroup analysis
Subgroup analyses in both TAP reports, presented in Table 2 suggested that the composition of the CNV lesion determines the extent of benefit from verteporfin therapy. At the 12-month follow-up, eyes that consisted of predominantly classic subfoveal CNV lesions at baseline (where the area of classic CNV occupies ≥ 50% of the area of the entire lesion) were the only subgroup of patients that appeared to benefit from verteporfin therapy. No treatment benefit was observed in eyes that consisted of minimally classic lesions at baseline (where the area of classic CNV occupies between 50% and 0% of the area of the entire lesion).
At 24 months of follow-up, the treatment benefit continued to be limited to those with predominantly classic lesions; no benefit was observed in those patients with minimally classic lesions. The treatment effect was even stronger for the subgroup of lesions composed of entirely classic CNV with no occult CNV at baseline. However, it is important to note that, even when study eyes with 100% classic CNV were removed from the predominantly classic CNV subgroup, patients with predominantly classic CNV with some occult CNV still experienced a benefit from verteporfin treatment. Additional analyses suggested that the difference in observed effects might have been due to baseline differences in visual acuity and overall lesion size between the predominantly classic and minimally classic subgroups.
Table 2: TAP Study: - Subgroup analysis by CNV lesion type
| Outcome |
Endpoint |
Predominantly Classic Lesion (≥ 50% of lesion is classic) |
Minimally Classic Lesion (>0% but <50% of lesion is classic) |
|---|
| |
|
V |
P |
|
V |
P |
|
| Loss of < 15 letters (primary efficacy outcome) % of eyes |
12 month |
67.3% |
39.3% |
p<.001 |
55.9% |
55.3% |
p=.92 |
| 24 month |
59.0% |
31.0% |
p<.001 |
47.5% |
44.2% |
p=.584 |
V = verteporfin, P = placebo
b) Verteporfin In Photodynamic Therapy Study (VIP).7
The VIP study involved 339 patients from 28 clinical centers in North America and Europe. The study’s purpose was to determine if OPT with verteporfin could safely reduce the rate of vision loss in patients with AMD-associated subfoveal CNV that were not included in the TAP study. Thus, the VIP study enrolled patients who had occult but no classic CNV on flourescein angiogram; or presumed early-onset classic CNV with baseline visual acuity of 20/40 or better. 258 subjects (76%) had occult with no classic CNV and 81 (24%) had early onset classic with good vision.
The study’s primary endpoint was the percentage of eyes that suffered moderate vision loss, as compared to baseline, at the 12-month follow-up. Moderate vision loss was, as in the TAP trial, defined as loss of 15 or more letters on a standardized eye chart. This corresponds to a loss of approximately three lines of vision from a standard Snellen eye chart. Of the 258 eyes in the occult with no classic subgroup, 166 eyes received treatment with verteporfin and 92 eyes received treatment with placebo. Of the 81 eyes with early classic CNV and good vision, 59 were treated with verteporfin and 22 were in the placebo group.
For the entire study group, the results did not achieve statistical significance at the primary endpoint (see Table 3). By 24 months, the entire study group of all eyes treated with verteporfin did show a benefit in terms of moderate vision loss (Table 3).
Mild adverse events, thought to be related to treatment, were noted in 43% (96/225) of the verteporfin group (both occult with no classic and early onset classic) and 18% (21/114) of the placebo group. These adverse events consisted of slight visual disturbances unsubstantiated on exam, injection site related problems, infusion-related back pain, allergic reactions, and photosensitivity. None of these events were considered to be serious.
A more serious adverse event, severe vision loss, was noted in ten of the 225 verteporfin-treated patients (4.4%) within seven days of treatment. None of the placebo patients experienced severe vision loss. This vision loss was characterized as at least a 20-letter decrease in visual acuity as compared to pretreatment acuity. Eight of these 10 patients had occult with no classic CNV at baseline. Thus, severe vision loss was seen in 4.8% (8/166) of the occult with no classic subgroup. By three months after this event, five of the 10 patients recovered vision to less than 20 letters lost as compared to their pretreatment acuity. According to the study investigators, some of these five patients who regained vision were in the occult with no classic group. It should be noted that severe vision loss in the TAP study was noted in only 1% of verteporfin-treated patients. The reason for this elevated risk of severe vision loss in the VIP study is unclear.
Table 3: VIP Study: Moderate and severe vision loss at 12 and 24 month follow-up, all study patients
| |
12 month Follow-up |
24 month Follow-up |
|---|
| |
Moderate loss |
Severe loss |
Moderate loss |
Severe loss |
| Verteporfin |
51% (114/225) |
24% (54/225) |
54% (121/225) |
30% (67/225) |
| Placebo |
54% (62/114) |
32% (36/114) |
67% (76/114) |
47% (54/114) |
| |
p=0.52 |
p=0.135 |
p=0.023 |
p=0.001
|
Subgroup analysis
The VIP study reported several subgroup analyses. As Table 4 shows, the subgroup of occult with no classic CNV did not show a statistical benefit in terms of verteporfin treatment at 12 months, the study’s primary endpoint. In addition, there was no statistically significant benefit for verteporfin treatment in terms of severe vision loss at 12 months. Treatment with the drug did reach statistical significance for both moderate and severe vision loss at 24 months.
Table 4: VIP Study: Moderate and severe vision loss at 12 and 24 month follow-up, occult with no classic subgroup
| |
12 month Follow-up |
24 month Follow-up |
|---|
| |
Moderate loss |
Severe loss |
Moderate loss |
Severe loss |
| Verteporfin |
51% (84/166) |
22% (36/166) |
55% (91/166) |
29% (48/166) |
| Placebo |
55% (51/92) |
33% (30/92) |
68% (63/92) |
47% (43/92) |
| |
P=0.51 |
P=0.07 |
P=0.032 |
P=0.004 |
c) Analyses considering both the TAP and VIP Studies
A recently published paper performed additional analyses using data from both the TAP and VIP studies.8 The authors noted a strong interaction between the treatment effect of OPT with verteporfin and lesion size for lesions that were minimally classic or occult only but not for predominantly classic lesions (Table 5). In the predominantly classic group, treatment appeared to reduce visual acuity loss by about 40%, regardless of lesion size. For subjects with minimally classic or occult with no classic lesions, significant treatment benefit was only seen in subjects with smaller lesions, less than 5 disk areas in size.
The size of the observed treatment benefit was proportionate to lesion size for predominantly classic lesions but inversely proportional to lesion size in minimally classic and occult but no classic lesions. In all verteporfin-treated patients, visual acuity loss was proportional to lesion size. This was also true for subjects with predominantly classic CNV who received placebo but not for subjects with minimally classic or occult with no classic lesions. Placebo-treated subjects with minimally classic or occult with no classic lesions suffered losses in visual acuity that diminished slightly with increasing lesion size.
Table 5. Loss of visual acuity at 24 months by lesion size and treatment group: TAP (minimally classic and predominantly classic) and VIP (occult only) studies
| |
Placebo |
Verteporfin |
|---|
| Lesion Size(disk areas) |
Occult Only |
Minimally Classic |
Predominantly Classic |
Occult Only |
Minimally Classic |
Predominantly Classic |
| 1 |
-23.4 |
-20.3 |
-16.3 |
-6.2 |
-8.5 |
-10.6 |
| 2 |
-22.9 |
-19.8 |
-19.5 |
-8.6 |
-10.3 |
-12.3 |
| 3 |
-22.4 |
-19.3 |
-22.7 |
-11.0 |
-12.0 |
-14.0 |
| 4 |
-22.0 |
-18.7 |
-25.9 |
-13.4 |
-13.8 |
-15.7 |
| 5 |
-21.5 |
-18.2 |
-29.1 |
-15.8 |
-16.5 |
-17.4 |
| 6 |
-21.0 |
-17.6 |
-32.3 |
-18.2 |
-17.3 |
-19.1 |
| More than 6 |
-20.1 |
-16.5 |
-38.6 |
-23.0 |
-20.8 |
-22.5 |
4. Medicare Coverage Advisory Committee
The Medicare Coverage Advisory Committee (MCAC) met on September 9, 2003, to discuss and make recommendations concerning the quality of the evidence and related issues for the use of OPT with verteporfin in routine clinical use in the population of Medicare beneficiaries who have AMD and occult with no classic CNV.
CMS staff presented the panel with information on AMD in the Medicare population, a history of Medicare coverage of verteporfin, and a review of MCAC voting questions and discussion questions. In addition, Dr. Charles P. Wilkinson presented the perspective of a practicing ophthalmologist, followed by a CMS review of evidence and data analysis.
Representatives from Novartis and QLT presented the panel with an overview of what macular degeneration is, how it can lead to decrease in vision, and ultimately to blindness. They explained various test and trial procedures and results from the TAP and VIP trials, and responded to the points raised in the CMS presentation. They presented new exploratory analyses that combined data from the TAP and VIP studies that suggested that response to OPT with verteporfin in patients with minimally classic or occult but no classic lesions was inversely proportional to lesion size. Some of the panel members suggested that this new evidence could alter their responses and requested a reconvening of the panel at a latter date to allow time to review this evidence. However, the majority of the panel voted to review only the evidence submitted prior to the meeting.
Representatives from the American Society of Retinal Specialists, the American Academy of Ophthalmology, and the American Council for the Blind addressed the panel, supporting national coverage for the requested treatment. Medicare recipients also related personal experiences with macular degeneration to the panel.
Thirteen scheduled public speakers addressed the panel. Representatives from Genaera and Genentech informed the panel of studies currently underway addressing possible treatment of macular degeneration being sponsored by their companies. Representatives of the Gray Panthers, the Seniors Coalition, the Baltimore Office of the NAACP, the American Association of People with Disabilities, the League of United Latin American Citizens, Lighthouse International, and Prevent Blindness America addressed the panel. Three Medicare beneficiaries and one other individual patient, all of who had received or were receiving verteporfin therapy related their personal experiences to the panel.
Five additional speakers addressed the panel during a period of open public comment, including the daughter of a Medicare beneficiary who had been treated with verteporfin, a representative from “Advancing Independence” and “Modernizing Medicare and Medicaid,” the spouse of a Medicare beneficiary who had been treated with verteporfin, an attorney representing Novartis and QLT, and a retired physician who had received verteporfin therapy.
The panel then engaged in a question and answer session with representatives of the requestor and CMS. Following this discussion, the panel voted on the following analytic questions:
- Is there adequate evidence to draw conclusions about the net health outcomes (that is, whether or not the risks and benefits of treatment outweigh the risks and benefits of non-treatment) of ocular photodynamic therapy (OPT) with verteporfin in routine clinical use in the population of Medicare beneficiaries who have age-related macular degeneration (AMD) and occult with no classic choroidal neovascularization (CNV)?
- If the panel answers the first question affirmatively, does the evidence demonstrate that OPT with verteporfin treatment improves net health outcomes in treating age-related macular degeneration (AMD) and occult with no classic choroidal neovascularization (CNV), and if so, what is the size of the benefit in patients receiving the treatment?
The results of the vote for the first question were eight yes, one no, and one abstention. The results of the vote for the second question were also eight yes, one no, and one abstention. The MCAC panel then considered the size of the benefit in patients receiving OPT with verteporfin for occult with no classic AMD. When compared with no treatment, seven voted the treatment to be more effective, one voted the treatment to be substantially more effective, and two panel members abstained.
5. Professional Society Position Statements
The American Academy of Ophthalmology (AAO) has published Preferred Practice Patterns for Age-Related Macular Degeneration8. The AAO Preferred Practice Patterns are published as a service to members of the Academy and to the public and are considered guidelines rather than definite standards of practice. With respect to OPT for patients with subfoveal choroidal neovascularization, the document includes the following statements:
New lesions containing classic CNV: Consider photodynamic therapy (PDT) for lesions that are predominantly classic CNV.
Recurrent lesions with evidence of classic and well-demarcated CNV: Consider PDT for recurrent lesions that are predominantly classic CNV.
New or recurrent lesions with evidence of classic and occult CNV: Consider PDT for lesions that are predominantly classic CNV and are less than or equal to 5400 microns in greatest diameter.
Occult but no classic CNV: Consider PDT, particularly if the lesion size is relatively small (< 4 MPS disc areas) or lower levels of visual acuity (approximate Snellen equivalent worse than 20/50).
6. Public Comments
CMS received numerous letters in favor of expanding coverage of verteporfin for the requested indication. These included letters from elected officials on behalf of their constituents, patients with AMD, caregivers, and advocacy organizations.
VIII. CMS Analysis
National coverage determinations (NCDs) are determinations made by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act § 1869(f)(1)(B). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be “reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member.” § 1862(a)(1)(A).
This section summarizes the agency’s critical appraisal of the evidence available on the effect of OPT with verteporfin on net health outcomes for Medicare beneficiaries. We address the questions that guided the overall assessment and the related evidence that led to the coverage conclusions.
The scientific validity of information obtained in the TAP and VIP studies can only be properly understood after a thorough assessment of their methodological quality. Careful examination of the protocols for both trials revealed important questions that affect the interpretation of the data, particularly in the VIP study.
Prespecification of the Analyses
Both the TAP and VIP studies fall short of the ICH guidelines described earlier in this document regarding the need for prespecification of the analysis. Both studies introduced new statistical analysis plans that differed substantially from those described in the initial protocols at a time when all subjects had already completed one year of treatment, the principal evaluation time point. The VIP analysis was also revised a second time three months after the last subject had completed two years of treatment. In both studies the initial protocols did not give specific criteria for categorization of variables, the splitting of the population into subgroups, or ranges of values.
The statistical plans establish the standards of proof to be used in the experiment. The accuracy and interpretability of statistical tests depends upon the specification of the variables to be tested, any categorization of variables, the methods for testing the significance, and the statistical power of those tests. These should all be determined before the experiment has begun. If any information resulting from the conduct of the experiment has the effect of altering the statistical plan, the results may become biased.
A study’s primary objective is to test the pre-defined hypothesis at its completion. The results of that test form the conclusions that can be drawn from that experiment with a high degree of confidence. Other analyses are useful in gathering insight into what factors may have contributed to the experimental results, generating hypotheses that could be tested in future experiments. Such subgroup analyses usually require additional confirmation since they increase the probability that associations between patient characteristics at baseline and health outcomes will achieve, or fail to achieve, statistical significance on the basis of chance alone.
The TAP study consisted of two separate simultaneous clinical trials under the same protocol. The null hypothesis to be tested in TAP was that the proportion of patient responders with regards to visual acuity was the same for verteporfin and placebo, whereas the alternative hypothesis was that the proportion of patient responders was different between verteporfin and placebo.
The original protocol described two primary outcome variables–loss of 15 lines and loss of 30 lines of visual acuity. It also specified two statistical tests for each variable, one that stratified for treatment center and two levels of baseline acuity and another that did not consider treatment center but stratified for eight levels of baseline visual acuity. The result was eight different indicators of whether the null hypothesis or the alternative hypothesis was the correct conclusion. The protocol gave no indication of how to account for multiple comparisons if these indicators were not in unanimous agreement. The revised analysis plan reduced the number of indicators to two (one for each trial, TAP 1 and TAP 2) but again did not specify how to reconcile a difference in results between the trials. Since both indicators showed statistical significance (p<0.02), this issue was moot in this study.
In addition, the published analyses from these trials differ substantially from those specified in the study protocols, particularly in their emphasis on subgroup analyses and the results at 24 months. The subgroup analyses cited in the papers were few among hundreds described in the initial analysis plans and were not given any special prominence in the original study protocols. These analyses cannot be considered definitive proof of the efficacy of OPT; they should only be considered to be sources of hypotheses for future clinical trials. The study protocols specify that the 24-month analyses were originally intended to confirm the durability of any effect seen at 12 months.
OPT with verteporfin did not reach statistical significance for preventing moderate (the primary outcome) or severe (a secondary outcome) vision loss at 12 months in the VIP trial. Thus, according to the protocol, the conclusion is that the null hypothesis (that the proportion of patient responders for visual acuity is the same for verteporfin and placebo at 12 months) cannot be rejected.
Unlike the TAP study, the 12-month results of the VIP study were not published until the 24-month results were available. Contrary to the analysis plan, the published VIP paper emphasized the results in a subgroup (subjects with only occult choroidal neovascularization) at 24 months as the principal findings of the study rather than as an exploratory examination of possible reasons for lack of apparent benefit at 12 months.
Post-hoc subgroup analysis in the VIP Study
The VIP study enrolled patients with CNV due to pathologic myopia (who did not have AMD) and the two groups of patients with AMD-related CNV that were excluded from the TAP trials. The TAP study excluded the following two groups of patients with AMD and CNV:
- Patients with AMD with occult CNV but no evidence of classic CNV.
- Patients with classic CNV with visual acuity better than 20/40
The investigators reported that patients in the first group had not been included in the TAP trial because “[v]isual acuity may deteriorate more in patients with lesions containing classic CNV than in patients with lesions containing occult with no classic CNV.” Inclusion of these patients might have delayed detection of a positive effect of treatment on classic CNV. With regard to the second group, “with limited safety data at the initiation of the TAP trial, the investigators were unwilling to apply this therapy to affected eyes with excellent visual acuity.” 9
Those VIP subjects with pathologic myopia were treated effectively as if they constituted a separate trial; they had separate power calculations, randomization, primary hypothesis and analyses. In contrast, despite differences in the characteristics of their disease and the reasons for exclusion, the VIP study protocol treated the two groups of patients with AMD as homogeneous. The primary hypothesis and power calculation for the study were based upon the entire population, and there was no attempt to stratify treatment assignment. The protocol did not specify a subgroup analysis that would separately look at the two groups. Instead, the protocol states: “Additional subgroup analyses will be made to evaluate any effect on outcome of CNV lesion size, lesion components, visual acuity and evidence of CNV in fellow eye, use of [indocyanate green angiography] and recurrent versus new lesions.” Thus, subgroup analyses of such effects are post-hoc and cannot provide definitive evidence for or against a possible benefit of therapy.
Masking
The protocols for both studies specified that the treatment assignment, placebo or active drug, be masked from patients, treating ophthalmologists, and visual acuity examiners. However, the study design made repeated disclosure of treatment assignment likely. In order to prepare treatment, the study coordinator or a designate needed to look up a subject’s group assignment every time the subject was treated (up to eight times) and follow a different process of preparation depending on treatment assignment. Furthermore, while the placebo was colorless, verteporfin has a distinctive color that would be readily noticeable. This process made it difficult to protect against excessive curiosity or inadvertent disclosure. For these reasons, the masking process may not have been very effective. Because the assessment of visual acuity is dependent upon the subject’s effort, the lack of an effective masking method may have skewed the results.
Approach to Missing Data
The analyses in both trials used a “last observation carried forward” (LOCF) approach to account for missing patient data. This method of data analysis assumes that there is no further change in vision from the time the participant was lost to follow-up until the endpoint. This assumption does not seem valid given the natural history of progressive visual loss with subfoveal CNV and could bias the results of the study in favor of the group with more dropouts. In the VIP study, for subjects with occult only CNV, the dropout rate (the proportion of subjects without a month 24 visual acuity measurement) for the active treatment group was 13.9% and 10.7% in the placebo group. Although this difference is not statistically significant (so we cannot be confident that this difference is not due to chance), the existence of this difference nonetheless constitutes a bias that is likely to favor the active treatment arm.
While it may be desirable to do an LOCF analysis for confirmatory purposes for submission to regulatory agencies, it is not ideal as the primary analysis in this context. The most conservative test would be to assume that subjects who are lost to the study are failures rather than successes. In the TAP study this did not influence the results. In the VIP study, if subjects lost to follow-up are considered treatment failures–a reasonable assumption considering the progressive nature of the disease and the apparent need for ongoing maintenance therapy–the difference between treatments in the overall group at 24 months is no longer statistically significant (p=0.064). However, the difference in the occult only subgroup does reach significance (p=0.043).
Choice of Primary Outcome
Another weakness in the design of both the TAP and VIP studies is the choice of primary outcome. Both studies used the loss of 15 or more letters of visual acuity after one year. This indicator limits the assessment of effect to a single threshold at a single point in time. In doing so, much useful information is ignored and conclusions can be influenced by random fluctuations occurring at the particular time or threshold. It gives limited insight into vision preservation over the course of treatment and what this means in terms of actual vision.
The use of indicators that are based upon thresholds or single points in time are most useful if the threshold (such as death) or point in time is uniquely important. This is not the case for AMD, as the thresholds are not unique. The loss in visual acuity from 14 to 16 letters cannot be considered more important than a loss from 6 to 14 letters or one from 16 to 25 letters. A treatment that led to inferior visual acuity for most of the year but a slight improvement at 12 months would not be considered superior. More information can be obtained by using measures of effect that do not rely on a fixed threshold or time point. Kaplan Meier survival curves, which were not presented in the published study, are useful in following data over time because they do not rely on predetermined intervals for looking at data. Indeed, in Kaplan Meier curves, events can be identified at the exact point in time at which they took place.
Figure 2.
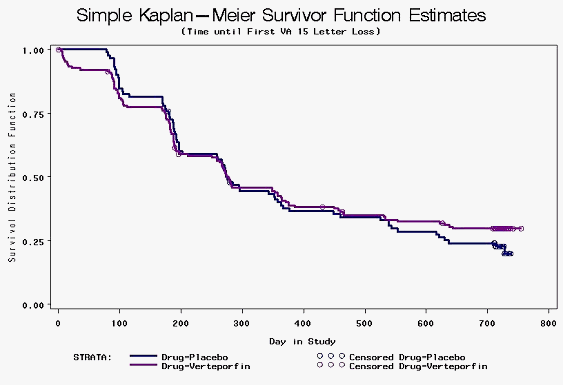
Figure 2 shows the curves for time to moderate (≥15 letter) vision loss comparing verteporfin and placebo in the occult with no classic groups in the VIP study. The time to ≥15 letter visual acuity loss curve shows that the placebo group did slightly better for the first six months while verteporfin showed a slight advantage after 18 months. On the whole, the difference between the curves is not statistically significant (p=0.551). Figure 3 shows the curves for time to severe (≥30 letter) vision loss comparing verteporfin and placebo occult with no classic groups in the VIP study. The time to ≥30 letter visual acuity loss curve shows little difference in the first year of the study while verteporfin showed some advantage after this point. On the whole, the difference between the curves is statistically significant (p=0.046).
Figure 3.
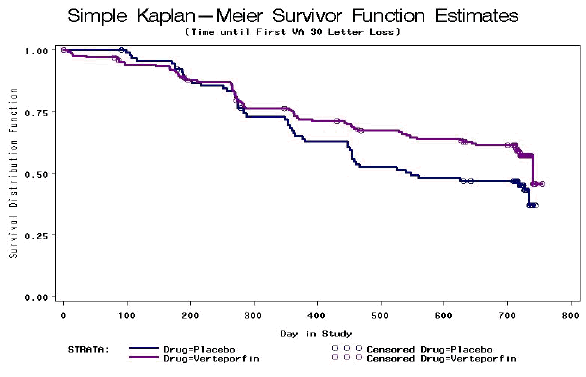
Alternatively, instead of looking at a specified threshold over time, visual acuity can be compared at a specified time point without specifying a threshold. Table 6a shows the results from the TAP study for average loss of visual acuity at 12 and 24 months. Table 6b shows the same figures for the VIP study for both the overall population and the occult only subgroup.
Table 6a. TAP Study: Average loss of visual acuity (Letters)
| Group |
Treatment Group |
12-Month Endpoint |
24-Month Endpoint |
|---|
| All Subjects |
Verteporfin |
11.3 |
13.4 |
| |
Placebo |
17.6 |
19.6 |
| |
|
p<0.0001 |
P<0.0001 |
Table 6b. VIP Study: Average loss of visual acuity (Letters)
| Group |
Treatment Group |
12-Month Endpoint |
24-Month Endpoint |
|---|
| All Subjects |
Verteporfin |
16.2 |
19.1 |
| |
Placebo |
20.0 |
25.1 |
| |
|
p=0.080 |
p=0.012 |
| Occult CNV Only |
Verteporfin |
15.7 |
19.0 |
| |
Placebo |
20.8 |
25.5 |
| |
|
p=0.045 |
p=0.017 |
By limiting the primary assessment point of loss of visual acuity to a single threshold and single time point, the investigators in both the TAP and VIP studies minimized the statistical power of the experiment, increasing the possibility that a random fluctuation would lead to a false positive or false negative result. The availability of other thresholds and time points such as severe loss of visual acuity at 24 months of treatment (as well as other more powerful methods comparing the treatment and control groups), allowed for additional comparisons that might appear compelling but lack statistical validity due to their post hoc nature.
Interpretation of the VIP Control Group
The TAP study showed that OPT with verteporfin was effective in treating classic CNV. The VIP study was not designed to distinguish the effect of treatment of occult CNV from that of treating patients with occult CNV who also presented with newly developing classic CNV. Any benefit seen in the occult CNV only subgroup might have been due to the development at some point during the trial of classic CNV. Thus, a positive result from the VIP study may simply be a repetition of the TAP findings, with benefits comparable to the TAP findings occurring in subjects who, without treatment, would have developed classic CNV and negligible benefits occurring in subjects who would not have developed classic CNV.
Flourescein angiography was performed every three months in the VIP study. With few exceptions, the results of the examinations are available only for the baseline, 12-month and 24-month visits. Among the 92 subjects in the control group who had occult only CNV at baseline, 39 (42%) had evidence of classic CNV recorded by the month 12 visit and 55 (60%) had classic CNV noted by the final examination. These numbers are large enough to plausibly attribute the observed treatment benefit to the treatment of newly developing classic CNV.
Treatment Effect and Lesion Size
The published conclusions of the TAP and VIP studies were that OPT with verteporfin was effective in treating predominantly classic CNV and occult CNV without classic CNV but not minimally classic CNV. These findings seemed inconsistent. Minimally classic CNV lesions always contain classic CNV and frequently contain occult CNV as well. If predominantly classic and occult with no classic lesions respond to OPT with verteporfin, minimally classic lesions would also be expected to respond.
This inconsistency was apparently resolved when treatment effects were analyzed according to lesion size. When stratified by lesion size, the treatment response observed for subjects with minimally classic CNV was similar to subjects with occult but no classic CNV. The observed difference in treatment effect between the two groups was due to the presence of larger lesions among subjects with minimally classic CNV.
This explanation leads, however, to another apparent paradox. For subjects with predominantly classic CNV, the observed treatment benefit from OPT with verteporfin was directly proportional to lesion size. Treatment of larger lesions led to a greater absolute benefit in terms of visual acuity loss prevented. For subjects with minimally classic or occult with no classic CNV, the observed treatment benefit was inversely proportional to lesion size. The greatest absolute benefit in terms of visual acuity loss prevented was in the treatment of the smallest lesions even though a larger lesion can be considered a collection of smaller lesions. Subjects with predominantly classic CNV who received placebo had a loss in visual acuity that increased steadily with lesion size. Subjects with minimally classic CNV or occult without classic CNV who received placebo had losses in visual acuity that were unrelated to lesion size or declined slightly with increasing lesion size. OPT with verteporfin seemed only to be of benefit to subjects with minimally classic CNV or occult without classic CNV who began the trial with smaller lesions.
It is not surprising that the observed response to treatment, when controlled for lesion size, was similar in the minimally classic and occult with no classic groups. The distinction between the two groups may be the presence of a small amount of classic CNV. It is also possible in this classification system for the lesion to contain very little of either form of CNV. Most of the lesion may consist of bleeding, scarring, or atrophy. In contrast, predominantly classic CNV lesions, by definition, consist mostly of classic CNV.
The therapeutic response of predominantly classic lesions in proportion to lesion size suggests that treatment benefit occurs as a result of its effect on the lesion itself and, specifically, its predominant component, classic CNV. This is supported by the lack of a similar response profile for minimally classic or occult with no classic lesions. In these subjects, classic CNV was either not present or present to a degree not so closely related to lesion size. In subjects with minimally classic or occult with no classic CNV, treatment response was likely not due to a direct effect on the lesions present at the onset of the study but to its effect on some subsequent development that was more likely to occur in subjects with smaller lesions than in subjects with larger lesions. As subjects with smaller lesions are more likely to be at an earlier stage in the natural history of “wet” AMD, they may have a greater potential for the development of classic lesions. OPT with verteporfin may be effective in these patients either because it prevents the development of classic CNV or because it treats classic CNV as it develops.
Conclusions
While there are methodological limitations in both the TAP and VIP studies, the VIP study has more serious methodological problems and the results are less robust. The evidence from the TAP trial that OPT with verteporfin is effective in the treatment of AMD with predominantly classic CNV is highly credible. We continue to have a sufficient level of confidence in this evidence to conclude that OPT with verteporfin is reasonable and necessary for this indication and will not alter our current national coverage decision for this indication.
The evidence provided by the VIP study concerning whether OPT is effective in AMD with occult CNV is more problematic. The trial was not originally designed to specifically examine the effect of OPT with verteporfin on occult CNV. The methods employed for masking and for the interpretation of missing data introduced potential biases that may have favored the treatment group. The null hypothesis of no treatment effect for the overall group was upheld at the study’s primary endpoint. Although additional data analyses did not consistently show a significant treatment effect, some did show a benefit from treatment with verteporfin in patients with occult AMD.
When controlled for lesion size, AMD with minimally classic CNV appears to be essentially the same clinical condition as AMD with occult but no classic CNV. Re-analysis of data from the TAP study indicated a possible benefit from OPT with verteporfin in patients who entered the trial with smaller lesions. The results of a similar analysis in the VIP trial show a similar benefit in this subgroup of patients.
Given that retrospective subgroup analyses are generally considered hypothesis generating, requiring confirmation in subsequent trials, we do not in general consider them to be sufficient evidence of efficacy to justify coverage. CMS considers the existing scientific evidence for the benefit of verteporfin in occult AMD to be qualified. This assessment of the evidence is consistent with the deliberations of the MCAC, which expressed numerous reservations about the VIP study, but ultimately voted 8-1 that the evidence was adequate to conclude that OPT with verteporfin improved net health outcomes in treating AMD in occult with no classic CNV. The MCAC vote was influenced in part by the new data on results of treatment in the subgroup of patients with smaller lesions. Recently revised clinical guidelines from the American Academy of Ophthalmology advise retinal specialists to consider treatment with verteporfin for occult without classic CNV for patients with small lesion size. In contrast, these guidelines clearly recommend treatment with verteporfin for patients with classic CNV, again reflecting the more qualified evidence in patients with occult without classic CNV.
In spite of some uncertainty regarding the benefits of this treatment, CMS considers the scientific evidence to be adequate to conclude that this treatment is reasonable and necessary for the specified subgroup of patients. While not definitive, there is strongly suggestive evidence for preserved vision in a significant fraction of treated patients, for whom no other treatment alternative currently exists. The clinical trial necessary to further evaluate this treatment is underway, but will not have results in the near future. Input from the MCAC and from the relevant professional association is supportive of making this treatment available.
Therefore, we believe that the totality of the evidence, in the context of the considerations outlined above, is sufficient to conclude that OPT with verteporfin is reasonable and necessary for Medicare beneficiaries who have AMD with associated CNV lesions that are either minimally classic or occult, that are four (4) disk areas or less in size, and have evidence of progression. We intend to issue a national coverage determination for this indication.
1 Elman MJ, Fine SL. "Exudative age-related macular degeneration." Ryan, SJ, editor: Retina. C.V. Mosby. St. Louis, MO. 1989
2 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline E9: Statistical Principles for Clinical Trials. Accessed at http://www.ich.org/pdfICH/e9.pdf. See also Federal Register, Vol. 63, No. 179, September 16, 1998, page 49583.
3 Hulley et al. Designing Clinical Research. 2001
4 British National Coordinating Centre for Health Technology Assessment. Clinical effectiveness and cost utility of photodynamic therapy for wet age related macular degeneration: a systematic review and economic evaluation. March 2003
5 Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. “Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report 1.” Archives of Ophthalmology 1999;117:1329-1345.
6 TAP Study Group. “Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials—TAP report 2.” Archives of Ophthalmology 2001;119:198-207.
7 Verteporfin in Photodynamic (VIP) Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-verteporfin in photodynamic therapy report 2. Archives of Ophthalmology 2001;131:541-560.
8 Treatment of Age-related Macular Degeneration with Photodynamic Therapy and Verteporfin in Photodynamic Therapy Study Groups: “Effect of lesion size, visual acuity, and lesion composition on visual acuity change with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report No. 1. American Journal of Ophthalmology. September 2003.
9 Letter from QLT, Inc., to CMS. February 4, 2002.


