To: File: CAG-00041N
Continuous Subcutaneous Insulin Infusion Pumps (CSII)
From: Grant P. Bagley, MD, JD
Director
Coverage and Analysis Group
John J. Whyte, MD, MPH
Julie K. Taitsman, MD, JD
Medical Officers
Coverage and Analysis Group
Subject: National Coverage Decision
Date: August 26, 1999
This memo serves four purposes: (1) outlines the description and treatment of diabetes mellitus; (2) reviews the history of Medicare's coverage policies on diabetes management; (3) analyzes the relevant scientific data related to the continuous subcutaneous insulin infusion (CSII) pump; (4) delineates the reasons supporting a positive national decision to cover the device for type I diabetics.
Description of Diabetes
A. Pathophysiology
Diabetes Mellitus is a disease of abnormal glucose metabolism characterized by a deficiency of insulin production, or by development of insulin-resistance, either of which results in abnormally high blood sugars. Diabetes Mellitus is generally subdivided into two categories: (1) Type I diabetes mellitus , (also known as insulin dependent diabetes mellitus [IDDM] or juvenile onset diabetes mellitus) and (2) Type II diabetes mellitus (also known as non insulin dependent diabetes mellitus [NIDDM] or adult onset diabetes mellitus)1 Type I diabetes may begin at any age but onset typically occurs in childhood or adolescence. Type I diabetes results from an immune mediated destruction of pancreatic islet beta cells causing decreased endogenous secretion of insulin and necessitating exogenous insulin therapy to maintain euglycemia. Type II diabetes is marked by peripheral resistance to the effect of insulin rather than absolute insulin deficiency.
B. Epidemiology
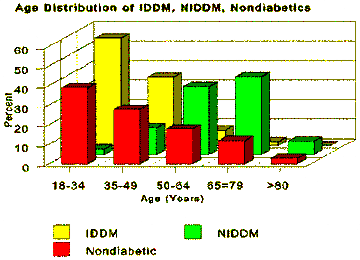
Approximately 16 million Americans have diabetes, although only slightly more than 10 million are diagnosed. Type I diabetes accounts for only a minority (about 5-10%) of the cases of diabetes, with an incidence of 30,000 new cases per year. Most new cases of Type I diabetes occur in patients under the age of 25 years. Diabetes significantly shortens life expectancy, and half of all Type I diabetics die before reaching age 50 years. The overwhelming majority of diabetics have type II diabetes, which has an incidence of 600,000 new cases per year. Type II diabetes is typically diagnosed in individuals over the age of 25 years, with almost half of all new cases occurring in people over age 55 years. Diabetes is especially significant in the Medicare population as more than 18% of persons over 65 years old have diabetes. (Only 2% of IDDM adults are over 65 years of age.)
C. Cost of diabetes
The acute and chronic complications of diabetes exert a dramatic toll on both the health care system and the morbidity and mortality of diabetes. Diabetes is the seventh leading cause of death in the United States, contributing to more than 193,000 deaths per year. Diabetic complications include retinopathy, nephropathy, neuropathy, and vascular complications. Diabetes is the leading cause of blindness, end stage renal disease, and non-trauma related lower extremity amputations. Additionally, diabetics have a two to four-fold increased risk of cardiac disease and stroke than non-diabetics. It is estimated that diabetes is responsible for $96 billion in direct medical costs and lost productivity each year. The long-term complications are the largest cost-driver. Diabetics spend about 24 million days in the hospital each year and it is estimated that at least 7 million of these hospital days are necessitated by diabetic complications. For example, Type I diabetics are prone to develop diabetic ketoacidosis (DKA), a potentially fatal elevation of blood sugar, which accounts for 3% of all hospital discharges. Both type I and type II diabetics are predisposed to develop hypoglycemia, which results in 30,000 hospitalizations per year.
Diabetes Treatment
Numerous authors employ the terms "conventional therapy" and "intensive therapy" with different meanings. This memo will define conventional therapy for type I diabetes as one or two subcutaneous insulin injections per day. It will define intensive therapy as treatment aimed at achieving as close to normoglycemia as possible accomplished either by three or more daily insulin injections or continuous subcutaneous insulin infusion.
A. Conventional Treatment
Management of diabetes involves efforts to maintain blood glucose levels near the normal range. Since the discovery of insulin in the 1920s, insulin replacement has served as the cornerstone of treatment for type I diabetics. Treatment for type II diabetes is somewhat more varied. Some type II diabetics achieve adequate glucose control with measures short of insulin replacement (e.g. diet, exercise, oral medications). A major component of diabetes treatment involves controlled diet and exercise. Exercise facilitates glycemic control in two ways: in the short term exercise decreases immediate insulin requirements, and in the long term exercise combats obesity induced insulin resistance by promoting weight loss, especially important for the nearly 40% of Type II diabetics who are overweight. Many type II diabetics use oral medications (e.g. sulfonlyureas) to either stimulate the pancreas to secrete more insulin, or to decrease peripheral resistance.
Under conventional therapy, insulin replacement has been provided with subcutaneous injections of insulin once or twice each day. For most patients, this treatment by subcutaneous injections involves some combination of short acting regular insulin and other longer acting insulin preparations. Such dosing also requires frequent monitoring of blood glucose levels, usually by finger-stick.
B. Intensive Control
Within the past few years, "intensive therapy" for diabetes management has gained favor as it seems to offer the greatest hope of preventing diabetic complications. Intensive therapy refers to frequent delivery of exogenous insulin (usually by injection four times a day or alternatively by continuous infusion) to obtain tight control in the normal blood glucose range. The Diabetes Control and Complications Trial (DCCT)2, offered compelling evidence that intensive treatment achieving tight glycemic control reduces the occurrence of microvascular and neuropathic complications in patients treated before the development of advanced disease. This trial involved 1,441 Type I diabetics at 29 medical centers. On average, patients were followed for an average of 6.5 years (range 3-9 years) before the study was terminated. The study's principal outcome measure was retinopathy, but it also included data regarding renal, neurologic, cardiovascular, and neuropsychological complications as well as adverse effects from treatment.
The DCCT examined two cohorts, a primary prevention cohort with a complication-free disease duration of one to five years, and a secondary intervention cohort with a disease course of one to fifteen years, and the initial signs of diabetic complications. Subjects were randomly assigned to the experimental group receiving intensive therapy or the control group receiving conventional therapy. Subjects in the experimental groups followed an intensive therapy regimen aimed at achieving as close to normal blood glucose levels as possible. Intensive therapy subjects had a choice of two methods of delivery of exogenous insulin; either via three or more daily insulin injections or external pump. [By the end of the study, 42% of the experimental subjects were using insulin pumps]. Subjects assigned to conventional therapy took one or two subcutaneous insulin injections per day. The study's results showed members of the intensive therapy group to have statistically significantly less progression of diabetic complications than the conventional therapy group: reduction in nephropathy of 34% and 43% for the primary prevention and secondary intervention cohorts respectively; 76% and 54% reduction in retinopathy, 69% and 57% reduction in neuropathy (see Table 1). The study found no statistically significant differences in quality of life between members of the conventional and intensive therapy groups (based on a questionnaire). The study's results were so convincing of the benefits of intensive therapy that the independent data monitoring committee recommended early termination of the trial. As the evidence favoring intensive therapy accumulated, investigators could no longer legitimately encourage subjects to remain in the less effective conventional therapy group.
Table 1 : Results of DCCT
| Outcome Measured |
Difference Between Conventional Therapy Group and Intensive Therapy Group: Primary Prevention Cohort |
Difference Between Conventional Therapy Group and Intensive Therapy Group: Secondary Intervention Cohort |
Retinopathy (progression of retinopathy by 3 or more steps) |
76% decrease for intensive therapy (95% CI: 62-85%) |
54% decrease for intensive therapy (95% CI: 39-66%) |
Nephropathy (development of microalbuminuria) |
34% decrease for intensive therapy (p=0.04) |
43% decrease for intensive therapy (p=0.001) |
Neuropathy (development of an abnormal neurologic exam) |
69% decrease for intensive therapy (p=0.006) |
57% decrease for intensive therapy (p<0.001) |
The DCCT demonstrated that intensive therapy offers numerous advantages over conventional therapy by decreasing the development of many long-term diabetic complications. However, in the short-term, the DCCT suggests that intensive therapy may pose some increased risks over conventional therapy. Subjects in the intensive therapy group experienced approximately triple the incidence of severe hypoglycemia (defined as hypoglycemia requiring assistance from another person) compared to the control group (p<0.001). There was however, no statistically significant difference between intensive and conventional therapy groups for occurrence of DKA or changes in neuropsychological functioning. The increased risk of hypoglycemia prompted the DCCT authors to recommend caution in starting intensive therapy for patients with a history of severe hypoglycemia or hypoglycemia unawareness. Additionally, the DCCT study population excluded prospective subjects who already had advanced diabetic complications. Given that implementing intensive therapy is not risk-free, the authors caution; "The risk-benefit ratio with intensive therapy may be less favorable...in patients with advanced complications."
C. Continuous Subcutaneous Insulin Infusion Pump
Multiple daily insulin injections represented the only available method of tight glycemic control until the development of continuous subcutaneous insulin infusion (CSII) in the late 1970s. CSII attempts to more closely replicate the normal pattern of secretion of endogenous insulin by supplying insulin at a baseline rate augmented by pre-meal insulin boluses. CSII delivery systems involve a battery-powered pump which holds a reservoir of buffered regular insulin. The pump propels insulin from the reservoir through an infusion set into a catheter inserted in the subcutaneous tissue of the abdomen (or alternatively the thigh or hip). The CSII systems do not measure blood glucose levels or automatically adjust insulin delivery rates. For proper effect the CSII user must measure blood glucose several times per day and program the pump to deliver an appropriate basal rate and pre-meal boluses of insulin. Currently two companies manufacture insulin pumps: MiniMed Technologies of Sylmar, California and Disetronic Medical Systems of Minneapolis, Minnesota.
Potential complications of CSII may result from either the inherent effects of insulin or the method of delivery. The most frequent complication involves infection at the infusion site. Pump users generally leave infusion sets in place in subcutaneous tissue for one to three days, and as with any indwelling foreign substance, this may precipitate infection. Most infusion site infections do not progress beyond cellulitis although abscesses sometimes develop. The responsible organism is usually staphylococcus aureus and infection is especially prevalent in pump users who are carriers of this bacteria. Less frequent but more serious complications of CSII involve excessive or insufficient delivery of insulin resulting in hypoglycemia or hyperglycemia respectively. Insulin imbalances may occur with all means of insulin delivery including standard subcutaneous injection but CSII introduces some unique mechanisms by which inappropriate insulin dosing may occur unbeknownst to the pump user. In CSII mechanisms the syringe holding the insulin may become blocked, the infusion set dislodged, or the pump otherwise cease functioning without the pump user's knowledge, causing inadequate insulin delivery potentially leading to hyperglycemia and most-dangerously diabetic ketoacidosis (DKA). Alternatively, incorrect programming of the pump or inherent pump malfunction (sometimes called "pump runaway") may cause excessive insulin delivery resulting in hypoglycemia. Hypoglycemia severe enough to cause impaired cognitive function may occur with all types of insulin therapy and is estimated to affect up to 30% of type I diabetics. The pump devices employ some warning mechanisms to help users identify when they are not receiving the intended amount of insulin but pump users must primarily rely on frequent blood glucose monitoring and subjective symptoms to determine when hypoglycemia or hyperglycemia necessitate adjustment of insulin infusion or immediate glucose ingestion.
History of Coverage Process
Diabetes management has been discussed in the Coverage and Analysis Group at HCFA for some time. HCFA has strived to ensure that beneficiaries with diabetes have access to quality care. Most recently, the Balanced Budget Act of 1997 (BBA '97) expanded benefits to patients with diabetes. BBA '97 allowed coverage of glucose monitors and test strips for Type II diabetics as well as expanded the types of diabetes education programs eligible for Medicare reimbursement
Patient self management plays a critical role in the successful treatment of diabetes. Traditionally Medicare has paid for some but not all of the tools required for self management (see Table 2 ). Medicare benefits include glucometers, lancets, and glucose test strips. Medicare benefits currently do not include insulin or the syringes, jet injectors, pen-type injectors, or pumps used to deliver insulin. Patients employing a subcutaneous insulin infusion pump require somewhat different tools for self management than practitioners of multiple daily injections (MDI) or conventional therapy. Like MDI and conventional therapy adherents, pump users would require glucometers, lancets, and glucose test strips. Pump users would also require insulin, however, it is either regular or short-acting insulin. Often, such patients require less insulin to manage their diabetes. Most significantly, instead of insulin syringes (except for a few back up syringes to use in case of pump failure), pump users would require an insulin pump, (which manufacturers estimate to last seven years), and certain disposable supplies necessary for pump functioning including insulin reservoirs, infusion sets, sterile adhesive dressings, and batteries.
Table 2: Supplies used in diabetes treatment by conventional therapy, MDI, or CSII
| Supply Item |
Needed for conventional therapy? |
Needed for MDI? |
Needed for CSII? |
Current Medicare Coverage? |
Lancets |
Yes, up to 1 or 2 per day |
Yes, 3 or more per day |
Yes, 4 or more per day |
Yes, under DME supplies |
Glucose test strips |
Yes, 1 or 2 per day |
Yes, 3 or more per day |
Yes 4 or more per day |
Yes, limited to 3 per day3 |
Glucometer |
Yes |
Yes |
Yes |
Yes |
Insulin |
Yes, regular and other longer acting preparations, unit requirements vary |
Yes, regular and other longer acting preparations, unit requirements vary |
Yes, regular insulin, unit requirements vary |
No |
Insulin syringes |
Yes, 1 or 2 per day |
Yes, 3 or more per day |
No (except for backup) |
No |
Insulin infusion pump |
No |
No |
Yes, one pump estimated to last 7 years (some users keep an auxiliary pump for backup) |
No |
Insulin reservoirs |
No |
No |
Yes, one reservoir every 4.5 days |
No |
Infusion sets |
No |
No |
Yes, one set every 3 days |
No |
Batteries |
No |
No |
Yes, one set every 6 weeks |
No |
Sterile adhesive dressings |
No |
No |
Yes, sufficient quantity to replace dressing every 3 days |
No |
The difference in supplies results in different costs for CSII vs multiple daily injections. Based on a cost estimate provided by Minimed and analyzed by HCFA staff, the annual cost for insulin pump therapy is approximately $3,329 (estimate includes amortized cost of pump over 7 years, pump supplies, insulin, and glucose test strips), vs the annual cost for MDI $1,817 (estimate includes syringes, glucose test strips, and insulin). The pump itself is the largest driver of the cost differential; greater market competition, however, would likely bring down the costs of the device.
Subcutaneous insulin infusion pumps fall under the benefit category of durable medical equipment (DME). Medicare coverage for most external drug infusion pumps is left to carrier discretion, however, regional carriers are currently explicitly prohibited from covering infusion pumps for insulin. Coverage Issues Manual Section 60-14 states "An external infusion pump and related drugs and supplies will be denied as not medically necessary in the home setting in the following situations: Insulin for the treatment of diabetes mellitus." This specific national noncoverage policy has been in effect since 1985.
In 1991, the Agency for Health Care Policy and Research (AHCPR) issued an assessment of insulin pump therapy.4 The AHCPR assessment stated that "the overall clinical evidence indicates that CSII is as effective as MDI in attaining normoglycemia in patients with insulin dependent diabetes mellitus who require intensive insulin therapy." In addition, the report noted: "Results from a number of controlled clinical trials have shown that CSII devices are effective in providing near-normo-glycemia and in improving metabolic control in patients with IDDM there is as yet no evidence to show that CSII is superior in clinical efficacy to MDI." AHCPR cautioned that "any form of intensive insulin therapy is also contraindicated for individuals with hypoglycemia unawareness and those with untreated preproliferative or proliferative retinopathy." Although the report noted that CSII poses risks of DKA, hypoglycemia, and skin infections, AHCPR suggested that these risks might be ameliorated as the technology improves.
In October, 1994 ECRI, a technology assessment firm based in Plymouth Meeting, PA, completed an assessment of CSII pumps.5 ECRI concluded that (1) insulin pump therapy produces greater metabolic control than conventional therapy (2) insulin pump therapy may produce greater metabolic control than intensive injection therapy. (3) the success of insulin pump therapy depends heavily upon proper patient selection, which in turn, depends heavily upon patient motivation. Regarding risks of severe hypoglycemic events, ECRI suggested that CSII might offer a decreased risk compared to MDI but that this is unproven "even though it seems that fewer severe hypoglycemic episodes are observed during insulin pump therapy than during intensive injection therapy, it would seem clinically prudent to assume that the number of these episodes in these two treatment types is equal." Of note, ECRI recommended caution in starting pump therapy on elderly patients because they may have difficulty responding to the warning symptoms of hypoglycemia.
HCFA reconsidered its position based on new data as well as the AHCPR and ECRI reports. Reconsideration of the issue of coverage for insulin pumps was raised at the Technology Advisory Committee (TAC) meetings of March 26-27, 1996, and August 6-7, 1996. At the March meeting the TAC discussed CSII and concluded that "there is no justification for coverage of this service." The TAC further decided that CSII lacked clinical evidence of effectiveness and could not be classified in a coverable benefit category and as such deemed issuance of a national policy unnecessary. CSII was further discussed at the August meeting and the TAC expressed concern that CSII poses potential dangers while its potential benefits have not been proven for the Medicare population. The TAC concluded that "the scientific data were not sufficient to demonstrate that the infusion pump could provide an effective administration of insulin to any patient in (the) Medicare or non-Medicare population."
Currently 153 private insurers and all but three state supplemental insurance organizations provide some coverage for CSII.
Recent Developments and Timeline of Activities
HCFA staff met with representatives of MiniMed in Spring, 1999 to discuss reconsideration of the national noncoverage policy for CSII.
In May, 1999 MiniMed sent materials which they believed supported a positive national coverage decision. MiniMed provided HCFA with a binder of materials dated May 7, 1999. This binder contained articles from the medical literature which are listed in Appendix B. The binder also contained letters supporting Medicare coverage of CSII, testimonials from satisfied pump users, and a list of private insurers and state supplemental insurance organizations currently covering CSII.
HCFA staff supplemented the information provided by MiniMed by independently conducting an extensive literature review. Full cites for the additional studies found are listed in Appendix B. HCFA's analysis of the studies provided by MiniMed or generated by HCFA is located in Appendix A. HCFA also reviewed the 1994 ECRI report on the CSII pump as well as the 1991 AHCPR report.
HCFA engaged in significant dialogue with MiniMed as well as their consultants, Patton Boggs, LLP. This dialogue included written, verbal, and face-to face meetings. Also, communicated with Disetronic Medical System.
On July 7, 1999 HCFA sent a letter to MiniMed with a series of questions relating to the literature. MiniMed responded with a letter dated July 23, 1999. MiniMed's letter included responses to HCFA's questions as well as additional articles from the medical literature. These articles are listed in Appendix B.
On August 12, 1999 HCFA met with representatives of the American Diabetes Association (ADA) and on August 19, 1999 met with representatives of the American Association of Clinical Endocrinologists to exchange scientific information regarding subcutaneous insulin infusion therapy.
Issues Related to Coverage of CSII
In reconsidering the national noncoverage policy for subcutaneous insulin infusion pumps, the following questions arise:
- Is intensive insulin therapy effective for treatment/management of diabetes?
- Is CSII an effective method of intensive insulin therapy?
- Are there significant differences in benefits between multidose insulin versus CSII?
- Are there significant differences in risks between multidose insulin versus CSII?
- What are the potential benefits of CSII in elderly diabetics?
- What are the potential risks of CSII therapy in elderly diabetics?
- Who is the appropriate patient population?
- What is the acceptance of this technology by the medical community?
Analysis of Scientific Data on CSII for Type I Diabetes 6
As mentioned earlier in the text of this Decision of Record, DCCT offers compelling evidence that intensive insulin therapy is effective for the management of diabetes. As Table I shows, the clinical benefits of decreased incidence/progression of retinopathy, nephropathy, and neuropathy can be seen in both the primary prevention and secondary prevention groups. Clearly, intensive insulin therapy is a goal for Type I diabetics.
The most common method of intensive insulin therapy is a multi-dose regimen of three or more daily insulin injections. However, continuous subcutaneous insulin infusion pumps are another method of achieving euglycemia. Is it an effective method? Numerous studies, including DCCT, document that CSII is an effective method of intensive insulin therapy. It lowers glycosylated hemoglobin and decreases incidence/progression of diabetic complications. The question invariably arises as to how does CSII compare to MDI as a method of intensive insulin therapy?
The DCCT offered strong evidence establishing the benefits of intensive therapy over conventional therapy, but only limited scientific data exists comparing the two methods of achieving intensive therapy, CSII and MDI. In the DCCT publications, for most outcome measures users of CSII and MDI were reported together, comprising the intensive therapy group, confounding attempts to compare the risks and benefits of CSII to MDI. The DCCT authors note the fact that intensive therapy patients were not randomly assigned to MDI or CSII and subjects could switch from one method of intensive therapy to the other implies that comparisons of the two outcomes for users of either group "will reflect patient, clinic, and treatment team differences as well as differences between the treatments themselves." Despite the preceding caveat, the DCCT does provide some valuable outcome measures to distinguish between CSII and MDI (see Table 3). Users of CSII achieved somewhat lower average levels of HbA1c indicating better glycemic control. Although the reported lower values for mean HbA1c levels do not independently demonstrate a clinical benefit of CSII, there is some evidence to suggest that any reduction in HbA1c offers some additional protection from developing diabetic complications. "Although the magnitude of the absolute risk reduction declines with continuing proportional reductions in HbA1c, there are still meaningful further reductions in risk as the HbA1c is reduced towards the normal range." This concept is often expressed by suggesting that there is no glycemic threshold for the development of long-term diabetic complications; meaning the goal for diabetes treatment should be to obtain as close to normal blood glucose levels as possible.
Another area where CSII may offer additional benefit over multiple daily injections is for those diabetics exhibiting "dawn phenomenon." Dawn phenomenon represents early morning hyperglycemia thought to result from insufficient nocturnal insulin. A study by Koivisto suggests that for diabetics using CSII, the dawn phenomenon may be prevented by programming the pump to increase the nocturnal rate of insulin infusion. Although little scientific data currently exists to prove the benefit of decreased early morning hyperglycemia, current beliefs regarding tight glycemic control suggest benefit of avoidance of hyperglycemia at any time.
In a study conducted by Bode et al on patients who had been on MDI and experienced poor glycemic control including severe hypoglycemia, the authors found that when patients switched to CSII, there were statistically significantly fewer episodes of severe hypoglycemia, and no difference in events of DKA. Of note, HbA1c was not different between the groups, in contrast to other studies which have documented decreased HbA1c for patients on CSII.
Table 3 : DCCT results specific for MDI and CSII
| Outcome |
MDI |
CSII |
CSII vs. MDI |
Catheter infections |
NA |
7.3 - 11.3 events per 100 patient years |
NA |
Mean HbA1c |
7.0% |
6.8% |
Lower for CSII (p<0.05) |
Hypoglycemic events requiring assistance (per 100 patient years) |
44 |
54 |
Difference not statistically significant
(no p value given) |
Hypoglycemic events resulting in coma or seizure (per 100 patient years) |
10 |
18 |
Higher for CSII than MDI (p=0.009) |
Episodes of DKA (per 100 patient years) |
0.8 |
1.8 |
Higher for CSII than MDI (p=0.045) |
A criticism of these studies relating to Medicare coverage, has been that DCCT as well as the majority of other studies, excluded elderly patients. Although studies on the Medicare population are not an absolute prerequisite to coverage, such studies do provide important information, since the Medicare population is often sicker, with significantly more co-morbidities than the non-Medicare population. In addition, the pathophysiology of disease is not always the same. Relating to the CSII pump, one must first realize that approximately 5,000,000 Medicare beneficiaries are below the age of 65 years (persons with disability and ESRD patients). Some of the beneficiaries could benefit from CSII. More importantly, the pathophysiology of diabetes is well-known. Although complications are often the result of length of diabetes , the nature of the disease itself does not change as people age meaning that Type I diabetes is not different pathophysiologically between young and old adults. In addition, there is a significant body of clinical experience documenting success for many elderly patients using the pump. There are a number of Type I diabetics less than 65 years of age who are doing quite well on the pump. The fact that they turn 65 years of age does not change the nature of their disease, nor their potential success using the pump.
HCFA recognizes that many of the Medicare beneficiaries with type I diabetes are likely to have more advanced diabetic complications than the populations studied. However, based on the consistency of the study results demonstrating benefit from any additional reductions in hyperglycemia in type I diabetics, it seems reasonable to extrapolate the data from the available studies to suggest a benefit of tight control in Medicare beneficiaries as well. The available studies comparing CSII to other means of obtaining tight control are limited, but some studies do suggest that CSII can offer tighter glycemic control than multiple daily subcutaneous insulin injections.
There has been some discussion about increased incidence of hypoglycemic unawareness in the elderly. Initially, several authors expressed concern that tight control would predispose patients to hypoglycemia. For those patients who had decreased awareness to hypoglycemia, either because of decreased autonomic response due to previous iatrogenic hypoglycemia or age, failure to take corrective action may cause such hypoglycemia to be life-threatening. CSII may actually decrease the frequency of hypoglycemic events compared to MDI. The pharmacokinetics of insulin delivery via CSII differs somewhat from subcutaneous injection. Subcutaneous insulin depots do not accumulate with CSII. Theoretically, this may diminish the risk of hypoglycemia by eliminating the phenomenon of sudden mobilization of accumulated insulin by such activities as exercise or other actions that increase blood flow. Recent studies, also have not substantiated earlier authors concerns regarding the use of CSII in patients with a history of hypoglycemia unawareness. In a study on patients with longstanding diabetes and a history of hypoglycemic unawareness, Cranston and others demonstrated that unawareness is reversible. Similar results were obtained in a study by Dagogo-Jack as well as Hirsch and Farkas-Hirsch. Physicians should exercise caution when they initiate an intensive insulin regimen in patients with a history of hypoglycemic unawareness, but it should not be a contraindication to the use of CSII. Within recent years, several authors have proposed hypoglycemic unawareness as an indication for CSII. Theoretically, the absence of an insulin depot may cause delivery of insulin via CSII to exert greater risk of ketoacidosis than conventional injections, but further studies are required before it can be asserted that this occurs in practice.
Opinions of Organizations Outside of HCFA
In the process of making this coverage decision, HCFA considered the positions of various groups with expertise in the field of diabetes. Several organizations have endorsed the use of CSII as a safe and effective means of obtaining tight glycemic control.
American Diabetes Association (ADA) Position:
The American Diabetes Association (ADA) is a non-profit organization primarily comprised of health care providers and researchers in the field of diabetes as well as people with diabetes. The ADA maintains a series of Clinical Practice Recommendations which are developed by experts based on scientific references and are subjected to annual peer review. The ADA endorses CSII in a current practice recommendation which states "Both CSII and multiple daily insulin injection therapy are effective means of implementing diabetes management with the goal of achieving near-normal levels of blood glucose ..... pump therapy is as safe as multiple-injection therapy when recommended procedures are followed."7 The ADA practice recommendation did not outline specific criteria for which patients are likely to benefit from CSII but it did note the effective use of CSII requires a motivated patient and may be too demanding for some patients. The ADA recommendation further noted that "in many people, CSII or multiple insulin injections can provide equivalent improvements in control" and explained that some clinicians only recommend CSII for patients for whom euglycemia has remained elusive on MDI while other clinicians offer CSII to patients without demonstrated failure of MDI but for whom conventional therapy is not commensurate with their lifestyle. The ADA neither endorsed nor disparaged either of these competing selection paradigms. HCFA discussed this clinical practice recommendation with several representatives of the ADA at a meeting on August 11, 1999. The published practice recommendation did not specifically address whether the recommendation was for type I or type II diabetics, however, the referenced citations were studies conducted on type I diabetics. At the August 11 meeting the ADA acknowledged the dearth of evidence for CSII in type II diabetics and expressed its opinion that insulin infusion pump therapy would be reasonable for type I diabetics in the Medicare population.
American Association of Diabetes Educators (AADE) Position:
The American Association of Diabetes Educators (AADE) is a non-profit organization comprised of health care providers in the field of diabetes, primarily those involved in diabetes education. The AADE also published an official organization position regarding insulin pump therapy.8 The AADE position statement asserts "given the results of the DCCT, CSII should be considered a treatment option because it offers increased lifestyle flexibility and enhanced self-management that improves blood glucose control. CSII is appropriate for individuals who (1) require or desire improved blood glucose control, especially during pregnancy; and/or (2) require the flexibility that CSII offers." The AADE position statement comments that hypoglycemia is a concern with CSII but frequent blood glucose monitoring can help avoid this problem. Like the ADA recommendation, the AADE position statement does not specifically address whether it refers to type I or type II diabetics but the references upon which it primarily relies involved studies on subjects with type I diabetes.
CSII is widely accepted in the medical community. As mentioned earlier, over 150 private insurers presently cover this device, including Kaiser-Permanente, Blue Cross Blue Shield, Prudential, and Aetna-US Healthcare.
Analysis of Scientific Data on Tight Glycemic Control and CSII for Type II Diabetes
The preceding scientific discussion focused on Type I diabetes. There are some studies which have tried to assert that tight glycemic control may help prevent the progression of diabetic complications in type II diabetics. Some studies of type II diabetes have compared "intensive" treatment with "conventional" treatment, but the terms reflected much different therapy than how they are usually used in type I. For example one study (UKPDS Lancet, 1998) found intensive treatment of type II diabetes to decrease the risk of microvascular but not macrovascular complications, and increase the risk of hypoglycemia. However, where in most of the type I studies, conventional treatment entailed insulin injections once or twice each day and intensive treatment entailed CSII or insulin injections three or more times each day, in the UKPDS study on newly diagnosed type II diabetics, conventional treatment involved only diet control and intensive treatment involved use of a sulfonylurea or any injected insulin. The study found that the intensive therapy group achieved lower mean HbA1c (7%) than the conventional therapy group (HbA1c 7.9%). The relevance of this study to the CSII coverage issue is minimal.
In a study conducted in Japan by Ohkubo et al on insulin-requiring type II diabetics, the authors found a difference in the incidence and progression of diabetic complications for those patients on intensive insulin treatment. However, the number of patients studied was small and no patients with advanced complications were included. In addition, no patients were on CSII. Of note, the authors state that "the benefit of intensive insulin therapy for Type II diabetics with advanced microvascular complications is not yet established."
The benefits of tight control in general or tight control employing CSII have not been proven for persons with type II diabetes. Both the body of scientific literature as well as clinical experience demonstrate that more research is needed for Type II diabetes (including insulin-requiring Type II): "It is not certain whether the findings from the DCCT regarding intensive insulin treatment for the control of hyperglycemia to prevent complications can be extrapolated to people with NIDDM... the risks and benefits of reduction of hyperglycemia in people with NIDDM need to be studied in a clinical trial before recommendations can be made.... While the current epidemiological data suggest that glycemic control is advantageous in people with NIDDM, care should be taken in using intensive insulin therapy to achieve such control, because the relation of the risks and benefits of such treatment is not known".9 Given the differences in disease physiology between type I and type II diabetes, proof of safety and efficacy for use of CSII for type II diabetics must be independently established in populations suffering from that disease, not extrapolated from studies of type I diabetics.
In conclusion, HCFA's analysis of the data suggests that CSII is a reasonable and necessary treatment of type I diabetes. Coverage for CSII should be limited to patients for whom the benefits are likely to outweigh the risks. The coverage policy would limit CSII to patients who have demonstrated ability to consistently and accurately monitor blood glucose at least three times per day and adjust insulin dosing accordingly. Coverage for CSII would also be limited to those patients who have a glycosylated hemoglobin > 7% at least 3 months prior to starting CSII.Coverage would continue to be denied for insulin-requiring Type II. As more data becomes available for those groups, HCFA will revisit the use of CSII for groups other than Type I diabetics.
DECISION:
Rescind the national noncoverage policy for external continuous subcutaneous insulin infusion pumps.
Amend Coverage Issues Manual 60-14 to add:
An external infusion pump and related drugs/supplies will be covered as medically necessary in the home setting in the following situation:
Treatment of Type I diabetes.
In order to be covered, patients must meet criterion A or B:
(A) The patient has completed a comprehensive diabetes education program, and has been on a program of multiple daily injections of insulin (i.e. at least 3 injections per day), with frequent self-adjustments of insulin dose for at least 6 months prior to initiation of the insulin pump, and has documented frequency of glucose self-testing an average of at least 4 times per day during the 2 months prior to initiation of the insulin pump, and meets one or more of the following criteria while on the multiple daily injection regimen:
(1) Glycosylated hemoglobin level(HbAlc) > 7.0 %
(2) History of recurring hypoglycemia
(3) Wide fluctuations in blood glucose before mealtime
(4) Dawn phenomenon with fasting blood sugars frequently exceeding 200 mg/dl
(5) History of severe glycemic excursions
(B) The patient with Type I diabetes has been on a pump prior to enrollment in Medicare and has documented frequency of glucose self-testing an average of at least 4 times per day during the month prior to Medicare enrollment.
Type I diabetes needs to be documented by a C-peptide level < 0.5
Continued coverage of the insulin pump would require that the patient has been seen and evaluated by the treating physician at least every 3 months.
The pump must be ordered by and follow-up care of the patient must be managed by a physician who manages multiple patients with CSII and who works closely with a team including nurses, diabetic educators, and dietitians who are knowledgeable in the use of CSII.
Subcutaneous insulin infusion pumps will continue to be denied as not medically necessary and reasonable for all Type II diabetics including insulin-requiring Type II diabetics.
1 Many people commonly, but mistakenly, label type II diabetics who use insulin as having insulin dependent diabetes. Such a characterization is a misnomer, since the physiology of the disease for such patients is different. Type II diabetics who use insulin will be referred to in this text as insulin-requiring Type II diabetics.
2 The Diabetes Control and Complications Trial Research Group published a series of articles including: "The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus". New England Journal of Medicine 329:977-986, 1993; "Implementation of treatment protocols in the diabetes control and complications trial", Diabetes Care 18:361-376, 1995; "Resource utilization and costs of care in the diabetes control and complications trial", Diabetes Care 18:1468-1478, 1995; "Lifetime benefits and costs of intensive therapy as practiced in the diabetes control and complications trial", JAMA 276:1409-1415, 1996. "The absence of a glycemic threshold for the development of long-term complications: the perspective of the diabetes control and complications trial", Diabetes 45:1289-1298, 1996.
3 Exceptions to the 3 per day limit may be granted upon proof of medical necessity and reasonableness.
4 AHCPR, "Reassessment of external insulin infusion pumps", DHHS publication No. AHCPR 91-0030.
5 ECRI, "Continuous subcutaneous insulin infusion pump therapy for diabetes", October, 1994.
6 Appendix A gives an overview of all articles reviewed.
7 American Diabetes Association, "Continuous Subcutaneous Insulin Infusion", Diabetes Care 22: Supp 1, pg s87, 1999.
8 "AADE position statement: education for continuous subcutaneous insulin infusion pump users", Diabetes Educator, 23:397-398, 1999.
9 Klein R, "Hyperglycemia and microvascular and macrovascular disease in diabetes", Diabetes Care, Vol. 18, No. 2, Feb 1995, pg. 258-268, pg. 265.


