To: File: Ocular Photodynamic Therapy with Verteporfin
CAG-00066N
From: Sean R. Tunis, MD, M.Sc.
Director, Coverage and Analysis Group
Svati B. Patel, MHS
Health Insurance Specialist, Coverage and Analysis Group
Poppy S. Rotter
Health Insurance Specialist, Coverage and Analysis Group
Michael Londner, MD, MPH
Medical Officer, Coverage and Analysis Group
Subject: National Coverage Policy Request
Date: November 8, 2000
This memorandum serves four purposes: (1) describes the pathophysiology of age-related macular degeneration; (2) discusses available treatments and recent developments in the clinical management of the disease; (3) analyzes relevant clinical literature on the use of ocular photodynamic therapy with verteporfin; and (4) delineates the reasons supporting national coverage of OPT with verteporfin for AMD patients with predominately classic subfoveal CNV lesions (where the area of classic CNV occupies ≥ 50% of the area of the entire lesion) as determined by a fluorescein angiogram.
Description of Age-Related Macular Degeneration
Age-related macular degeneration (AMD), a common ophthalmologic disease affecting the elderly, involves the deterioration of the central region of the retina called the macula, which results in a severe and irreversible loss of central vision. This vision loss can significantly impair and/or destroy visual acuity (the ability to distinguish visual details). However, although these individuals may progress to become legally blind, this retinal disorder rarely results in total blindness. Patients with AMD often maintain enough peripheral vision to walk unaided.
AMD is the leading cause of severe vision loss in the elderly in the United States, Canada, Britain, and Australia. The National Institutes of Health (NIH) estimate that nearly 1.7 million elderly Americans, 5% of the total population over 65 years of age, have some degree of vision loss due to AMD.1 Prevalence of this retinal disorder increases exponentially with age. For example, the rate of late-stage AMD among people 43 to 54 years of age is 0.1%. The prevalence of the disease increases to 7.1% for individuals 75 years of age or older.3 The underlying etiology of AMD is not well understood, however population-based studies have found several risk factors for the disease in addition to old age including family history of AMD, low dietary intake or plasma concentrations of antioxidant vitamins and zinc, and cigarette smoking. AMD has also been found to occur more frequently in Caucasians.3
The Macula
The retina is composed of a nine-layered sheet of highly organized photoreceptor cells which lines the back of the globe. This light sensitive tissue transmits signals via the optic nerve to the brain to be translated into visual images. In the central region of the retina, within its vascular arcades, is a circular area five mm to six mm in diameter known as the macula. The macula has the highest concentration of photoreceptor cells in the retina, enabling central vision and permitting high-resolution visual acuity. Central vision is essential in performing daily activities such as reading text, driving, and being able to distinguish facial features. The fovea is a specialized region at the center of the macula that provides the sharpest vision. This region of the macula, which functions in bright illumination, contains only cone disks (photoreceptor cells that facilitate central vision and color vision).
Figure 1
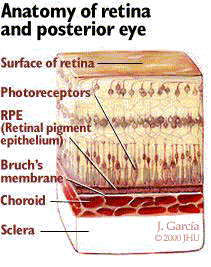
As illustrated in Figure 1, a single, villous layer of pigmented epithelial cells called the retinal pigment epithelium (RPE) is adjacent to the photoreceptor layer. Cone disks and rod disks (photoreceptor cells that facilitate peripheral vision and night vision), are contained in the villi of the RPE. The RPE’s primary responsibility is to nourish and maintain the photoreceptor layer of the retina. Beneath the RPE lies a membrane composed of acellular, collagenous, elastic lamina called the Bruch’s membrane. The Bruch’s membrane serves as a diffusion barrier between the RPE and the choroid, a highly vascular layer of tissue containing blood vessels. The photoreceptor layer and the RPE are continuously supplied with oxygen and nutrients by the choroid. The innermost layer of the choroid (the one closest to the Bruch’s membrane) is called the choriocapillaris, a single layer of closely spaced capillaries. Although most regions of the retina are also supplied with blood by other sources in addition to the choroid, the macula is exclusively supplied with blood by the choriocapillaris. This is of key importance in the pathophysiology of AMD.
Stages of Age-Related Macular Degeneration
AMD can be classified into early and late stages. Early stage AMD is associated with minimal visual impairment and the presence of drusen and pigmentary abnormalities in the macula. Drusen is yellowish, acellular debris that accumulates under the macula adjacent to the basement membrane of the RPE (the layer closest to the Bruch’s membrane). Drusen is characterized in two ways: small drusen and large drusen. Small drusen—also called "hard" drusen—are less than 50 μm in length and have distinct, well defined borders.4 Its presence is common among the elderly; nearly all people over 50 years of age have at least one area of accumulation. The presence of small drusen is not, however, considered sufficient for a diagnosis of AMD. Large drusen—also called "soft" drusen—are greater than or equal to 63 μm in diameter and have ill-defined, amorphous borders. Epidemiological studies show an increased risk of late-stage AMD associated with the presence of large drusen.5 Although the exact cause is unknown, experts believe that the presence of large drusen clinically corresponds to areas of diffuse, abnormal thickening of the inner aspect of the Bruch’s membrane (the section of the membrane closest to the RPE).6 Hypopigmentation within the RPE can develop in areas overlying this diffuse thickening. As debris continues to accumulate, the thickened inner aspect of the Bruch’s membrane and the overlying section of the RPE separate from the rest of the Bruch’s membrane, resulting in small RPE detachments.
The macula can be extremely prone to injury as a result of these detachments. When such damage occurs, the patient is believed to have progressed to late-stage AMD. There are two forms of late-stage AMD: atrophic and neovascular. The atrophic form (also known as the "dry" form) accounts for nearly 80% to 90% of all cases of AMD. The neovascular form (also known as the "wet" form) accounts for only 10% to 20% of all cases of AMD.
Atrophic ("Dry") Age-Related Macular Degeneration
The accumulation of large drusen and the associated abnormalities of the RPE are instrumental in the development of atrophic AMD. This form accounts for approximately 80% to 90% of all cases of AMD. As acellular debris continues to build up, the RPE and photoreceptor layers of the retina are lifted further and further away from the choriocapillaris. As its name implies, atrophic AMD involves the gradual deterioration of the photoreceptor layer, the RPE, and the choriocapillaris possibly resulting from a disruption of blood flow to the macula. The loss of the overlying photoreceptor layer translates into blind spots in the field of vision. As this disease progresses, these areas of vision loss coalesce into contiguous areas of atrophy. Large regions of RPE and photoreceptor cell loss, usually 175 μm or greater in length, are called geographic atrophy.8 Visual impairment due to atrophic AMD is slow and progressive, with vision loss occurring over a span of years. As described by Fine, et al. (2000), patients with atrophic AMD maintain fairly "good central vision (20/40 or better) but [have] substantial functional limitations, including fluctuating vision, difficulty reading because of their limited area of good central vision, and limited vision at night or under conditions of reduced illumination." Severe vision loss can result when photoreceptor cell deterioration begins to take place within the fovea. Despite being the most common type of AMD, the atrophic form accounts for only 12% to 21% of cases of legal blindness attributed to AMD.9 Most cases of severe vision loss are attributed to neovascular AMD.
Neovascular ("Wet") Age-Related Macular Degeneration
Visual impairment due to neovascular AMD results from the abnormal growth of new blood vessels which begin in the choriocapillaris and advance through the Bruch’s membrane into the RPE, a process known as choroidal neovascularization (CNV). This form accounts for 10% to 20% of all cases of AMD. Researchers have not determined the exact cause of this pathological growth of blood vessels. One theory suggests that the continued accumulation of large drusen detaches the RPE layer and a section of the Bruch’s membrane from the choroid, impairing the transport of oxygen and nutrients to the RPE and photoreceptor layer.10 In order to compensate for the obstructed blood flow to the macula, the choriocapillaris may be stimulated to produce and extend additional capillaries through the Bruch’s membrane into the RPE, thereby restoring the flow of nutrients and oxygen. Large drusen corresponds to a diffuse thickening of the inner aspect of the Bruch’s membrane. This thickening may either predispose the Bruch’s membrane to develop cracks, through which CNV can develop, or weaken the membrane’s diffusion barrier, allowing new vessels to break through the membrane and reach the RPE.11 Once they penetrate the Bruch’s membrane, the new choriocapillaris networks absorb the drusen and become imbedded in the epithelia layers of the RPE. These CNV vessels, which contain porous endothelial tubes, leak blood and protein fluid under the RPE causing the overlying area of the macula to bow upwards and shift it from its normal position, resulting in the formation of a lesion. Neovascular AMD is often called "wet" AMD because of the presence of this leakage. Fibroblasts (immature cells which differentiate into connective tissue) often accompany the growth of CNV vessels.12 If neovascular lesions are left untreated, these fibroblasts are stimulated by the collection of blood and fluid to replace normal anatomical structures in the macula, including the photoreceptor layer, with fibrovascular scar tissue (a process known as metaplasia), thus permanently disrupting the normal physiological architecture of the macula. This fibrovascular formation, called a disciform scar, is irreversible and can result in a permanent scotoma (blind spot).13 If such scar formation takes place in the fovea, the patient can experience a profound loss of central vision and visual acuity.
Although it is most often due to AMD, CNV can also be caused by other retinal disorders such as pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, or idiopathic causes. The development of CNV secondary to AMD should be suspected if an elderly patient complains of sudden metamorphopsia (a disturbance in vision in which objects appear distorted), scotomas, and non-specific changes in central vision.14 A definitive diagnosis can be made by fluorescein angiography. Neovascular lesions are classified as either classic or occult, according to their appearance on a fluorescein angiogram. Classic lesions are characterized by well-demarcated boundaries of bright hyperfluorescence. Occult lesions, however, do not have well-defined boundaries, often appearing as amorphous or obscured areas of fluorescence. Yet, only in rare cases are lesions considered entirely classic or occult. Most lesions resulting from CNV contain both classic and occult components. The histologic differences between classic and occult lesions have not yet been determined; thus far, definitions for these lesions and their identification are linked to their appearances on a fluorescein angiogram.
In contrast to the atrophic form, neovascular AMD causes damage to the macula and extensive central vision loss in a relatively short period of time, usually only over a span of a few months. Given its rapid disease progression, CNV is often the primary cause of severe vision loss seen in patients with AMD. For example, although the neovascular form is present in only 10% of all people with AMD, it is identified in 80% of patients who experience severe loss of visual acuity (20/200 or worse) due to AMD.15
Available Treatments & Recent Developments
Currently, there is no accepted treatment available to patients with atrophic AMD to help mitigate the extent of central vision loss. Two treatments, discussed below, are presently available to patients with neovascular AMD.
Laser Photocoagulation Therapy
Laser photocoagulation therapy is the only widely studied treatment available for patients with neovascular AMD. This technique involves the use of a strong light source targeted on CNV lesions in order to coagulate the diseased tissue. The laser produces a thermal effect within the lesion which causes necrosis of its cellular components. There are, however, three important limitations of photocoagulation therapy:
- The treatment benefits only a small number of patients with neovascular AMD. Patients with occult CNV and/or large lesions generally do not respond well to treatment.
- There is a high recurrence rate of neovascular lesion formation after the initial laser treatment (≥ 50%), most of which develop subfoveally (under the fovea).
- Although the laser is directed at the section of the macula containing the lesion, photocoagulation therapy is only partially selective. The thermal effect of the laser also damages viable photoreceptor cells overlaying the CNV lesion which can result in some degree of visual acuity loss depending on the area of the macula that has been treated. Patients with visual acuity of 20/200 or better prior to treatment, as well as patients who initially present with subfoveal CNV, are especially prone to a sharp, irreversible reduction in central vision.
Despite these limitations, studies have shown that the progression of vision loss in select patients with neovascular AMD (factoring in the vision loss resulting from the treatment itself) is less in laser-treated eyes than what would otherwise be seen in the natural course of the disease. Laser treatment is quite effective in patients with small, classic CNV lesions outside the fovea with visual acuity 20/125 or worse.16
Ocular Photodynamic Therapy
Recent developments have occurred in the clinical management of neovascular AMD involving a treatment called ocular photodynamic therapy (OPT). OPT for neovascular AMD involves the intravenous infusion of a photosensitive (light-activated) drug with a very specific absorption peak. This drug is chemically designed to have a unique affinity to the histologic characteristics of neovascular lesions. Intravenous infusion is followed by the targeted irradiation of the CNV tissue with a non-thermal laser calibrated to emit light at a wavelength that corresponds to the drug’s absorption peak. In theory, the drug then becomes active and locally generates reactive singlet oxygen and other reactive intermediates which can damage the cellular components of the adjacent CNV tissue—including cellular membranes, mitochondria, nuclei, and the vascular endothelium—resulting in the temporary thrombosis and closure of the leaking blood vessel. The most important benefit of OPT over laser photocoagulation is its ability to selectively destroy CNV lesions through the use of a non-thermal light source, resulting in minimal injury to the overlying photoreceptor layer.
Recent Developments and Timeline of Activities
On April 12, 2000, the Food and Drug Administration (FDA) reviewed and approved a drug called verteporfin (trade name Visudyne™) for the treatment of AMD in patients with predominantly classic subfoveal CNV. Subsequently, verteporfin was also approved for inclusion in the United States Pharmacopoeia (USP) on July 18, 2000, meeting Medicare’s definition of a drug as defined under §1861(t)(1) of the Social Security Act. Verteporfin, a benzoporphyrin derivative, is a lipophilic photosensitive drug with an absorption peak of 690 nm. Animal studies have shown that verteporfin demonstrates a selective affinity for neovascular lesions in the eye, making it an ideal drug for use in OPT to treat CNV.17 However, the thrombosis associated with OPT with verteporfin is only temporary; studies have shown that CNV blood vessels often begin leaking fluid again within three months of treatment, requiring the need for additional treatments after the initiation of OPT. It is suggested, though, that periodic treatments with verteporfin can minimize CNV leakage (thus lessening the extent of the associated loss of visual acuity) without damaging the photoreceptor layer of the retina.18
Verteporfin is the only photosensitive drug to be approved by the FDA for use in OPT to treat neovascular AMD, making it a significant therapy for this leading cause of blindness in the elderly. Given its importance as a major public health issue for the Medicare population, on July 26, 2000, the Health Care Financing Administration (HCFA) internally generated a national coverage review of OPT with verteporfin. There was considerable confusion among the Medicare contractors regarding the scope of coverage for this procedure/drug which the agency hoped to address with a consistent national coverage policy. The objective of HCFA’s coverage review was to determine whether this treatment was considered medically reasonable and necessary for patients with neovascular AMD and to distinguish, if possible, which patient population would benefit from this therapy from the available evidence.
HCFA held several meetings with various interested parties on September 6, 13, and 27, 2000, to discuss its coverage review of OPT and verteporfin.19 In addition, relevant background materials, as well scientific evidence, were submitted to the agency on September 7 and 15, 2000. Throughout the months of August and September, analysts from HCFA’s Coverage & Analysis Group (CAG) conducted extensive searches for scientific articles regarding OPT and verteporfin using electronic databases such as Medline and Ovid. Keywords used in these electronic searches included "macular degeneration", "choroidal neovascularization", "photodynamic therapy", and "verteporfin". Members of CAG also spoke with Dr. Neil Bressler, a principal investigator for the Treatment of AMD with Photodynamic Therapy (TAP) study, by phone to request additional background information on AMD and clarify information on the available clinical evidence. Although a number of journal articles were found regarding the treatment of neovascular AMD, HCFA focused its coverage review on articles that discussed the clinical effectiveness of OPT with verteporfin for neovascular AMD.
Evidence of Clinical Effectiveness
HCFA’s analysis of the scientific evidence was predominantly concentrated on the TAP trial at both the 12-month and 24-month follow-up.20 The TAP investigation was a large multi-center randomized clinical trial which was specifically conducted "to determine if photodynamic therapy with verteporfin…can safely reduce the risk of vision loss in patients with subfoveal CNV caused by AMD." The study was performed in 22 ophthalmology practices in North America and Europe (11 in the United States, two in Canada, and nine in Europe). Important inclusion criteria include the following:
- CNV must be the result of AMD,
- CNV must be under the geometric center of the foveal avascular zone (subfoveal),
- Patients must have evidence of some classic CNV (with or without occult component) as determined by fluorescein angiography,
- Best-corrected visual acuity of 73 through 34 letters (approximate Snellen equivalent of 20/40 through 20/200),
- Patients must be ≥ 50 years of age,
- Patients must be able to return for two years of follow-up.
Some of the important exclusion criteria were:
- Any ocular disease other than CNV that could impair (or has already impaired) vision in the study eye,
- Inability to obtain photographs to document CNV, including difficulty with venous access,
- Active hepatitis or clinically significant liver disease,
- Porphyria or other porphyrin sensitivity,
- History of treatment for CNV in the study eye other than nonfoveal laser photocoagulation.
For a complete listing of inclusion and exclusion criteria, please refer to Attachment B.
609 patients were randomly assigned to either the verteporfin treatment group (six mg of verteporfin per m2 of body surface followed by an application of a non-thermal laser for 83 seconds) or the placebo group (injection of 5% dextrose in water followed by an application of a non-thermal laser for 83 seconds) using color-coded sealed envelopes that were opened only after a patient was determined to have met the eligibility criteria. Neither the physician nor the patient was aware of or had any control over group assignment. Two-thirds of the study population were randomized to verteporfin treatment (n=402 eyes) while one-third were randomized to the placebo group (n=207 eyes). Randomization yielded balanced study groups. Extensive measures were taken so that patients, ophthalmologists, vision examiners, photographers, photograph graders, clinic monitors and sponsors were masked to treatment assignments and results.
The primary efficacy outcome of the TAP study was the percentage of patients (eyes) that had fewer than 15 letters lost (approximately three lines of visual acuity lost) compared to baseline at 12 months and 24 months. This value was chosen because a loss of three lines or more is indicative of moderate visual loss.21 Secondary efficacy outcomes included the following:
- Proportion of eyes that had fewer than 30 letters lost (approximately six lines of visual acuity loss) compared to baseline—a measure of severe visual acuity loss,
- Mean changes in visual acuity,
- Mean changes in contrast threshold,
- Angiographic outcomes (lesion size, leakage, and progression).
These secondary efficacy outcome measures were included to validate any observed changes in the primary efficacy outcome within and between the two treatment groups.
The aggregate results of the 12 and 24 month examination for the entire study population are given in Table 1. At 12 months of follow-up (TAP report 1), the verteporfin group, overall, had a lower risk of visual acuity loss compared with placebo. In addition, mean contrast sensitivity was better in the verteporfin group compared with placebo. Differences in angiographic outcomes between the two groups at 12 months reinforced the observed treatment benefit of verteporfin. The verteporfin group showed greater reductions in lesion size, leakage, and progression than the placebo group. The results of the 24-month examination (TAP report 2) corroborated those of the 12-month examination. At two years of follow-up, visual acuity and mean contrast sensitivity remained significantly better for the verteporfin group compared with placebo. The angiographic outcomes at the 24-month follow-up also continued to show improvements in CNV lesion size, leakage, and progression in the verteporfin group.
Despite the significant improvements in angiographic outcome measures and the slowed rate of visual acuity loss observed in the verteporfin group, some leakage from the treated subfoveal CNV lesion still occurred. Additional treatments were only administered to study participants if fluorescein angiography, which was performed every three months, revealed CNV leakage. If no leakage was detected, patients were not retreated. For the verteporfin group, patients were retreated an average of 3.4 times per participant during the first year of the study and 2.2 times during the second year of the study for a total of 5.6 treatments per participant over a two year period. In comparison, the placebo group was retreated an average of 3.7 times during the first year and 2.8 times during the second year for a total of 6.5 treatment per participant over two years. Neither TAP report 1 nor 2 addressed the issue of treatment cessation, an important concern given that an average patient may have to commit to nearly six treatments over a two year period. The appropriate frequency of treatment, the criteria needed to determine treatment failure, and the appropriate number of treatments needed beyond two years are questions that remain unanswered. Long-term follow-up studies would be helpful in addressing these questions.
Table 1: Analysis of Overall Treatment Effect
| Outcome |
Treatment Group |
12-Month Endpoint |
24-Month Endpoint |
|---|
Loss of < 15 letters
% of eyes |
V |
61.2 |
53.0 |
P |
46.4 |
38.0 |
p |
p<.001 |
p<.001 |
Loss of ≥ 30 letters
% of eyes |
V |
14.7 |
R/P |
P |
23.7 |
R/P |
P |
p<.001 |
R/P |
Mean # of contrast sensitivity letters lost |
V |
1.3 |
1.3 |
P |
4.5 |
5.2 |
p |
p<.001 |
p<.001 |
Fluorescein Angiography (Progression of CNV lesion)% |
V |
46.0 |
R/P |
P |
71.1 |
R/P |
p |
p<.001 |
R/P |
Fluorescein Angiography (Eyes with no leakage from classic CNV) % |
V |
18.8 |
R/P |
P |
9.1 |
R/P |
p |
p<.001 |
R/P |
V = verteporfin, P = placebo, p = p-value, N/A = not available, R/P = results pending publication
Subgroup analyses in both TAP reports, presented in Table 2, suggest that the composition of the CNV lesion determines the extent of benefit from verteporfin therapy. At the 12-month follow-up, eyes that consisted of predominantly classic subfoveal CNV lesions at baseline (where the area of classic CNV occupies ≥ 50% of the area of the entire lesion) were the only subgroup of patients that appeared to benefit from verteporfin therapy. No treatment benefit was observed in eyes that consisted of minimally classic lesions at baseline (where the area of classic CNV occupies between 50% and 0% of the area of the entire lesion). At 24 months of follow-up, the treatment benefit continued to be limited to those with predominantly classic lesions; no benefit was observed in those patients with minimally classic lesions. The treatment effect was even stronger for the subgroup of lesions composed of entirely classic CNV with no occult CNV at baseline. However, it is important to note that, even when study eyes with 100% classic CNV were removed from the predominantly classic CNV subgroup, patients with predominantly classic CNV with some occult CNV still experienced a benefit from verteporfin treatment.
Table 2: Subgroup Analysis of Treatment Effect by CNV Lesion Type
| Outcome |
Endpoint |
Predominantly Classic Lesion (≥ 50% of lesion is classic) |
Minimally Classic Lesion (>0% but <50% of lesion is classic) |
Classic with No Occult Lesion |
|
|
V |
P |
p |
V |
P |
p |
V |
P |
p |
Loss of < 15 letters (primary efficacy outcome) % eyes |
12 month |
67.3 |
39.3 |
p<.001 |
55.9 |
55.3 |
p=.92 |
76.6 |
30.6 |
p<.001 |
| 24 month |
59.0 |
31.0 |
p<.001 |
47.5 |
44.2 |
p=.584 |
70.0 |
29.0 |
p<.001 |
| Mean # contrast sensitivity letters lost |
12 month |
0.4 |
5.5 |
p<.001 |
2.0 |
4.1 |
p=.008 |
1.5 |
2.3 |
p=.65 |
| 24 month |
0.2 |
6.4 |
p<.001 |
N/A |
N/A |
V = verteporfin, P = placebo, p = p-value, N/A = not available, R/P = results pending publication
National Coverage Determination
The scientific evidence on OPT with verteporfin points to a significant benefit for a limited number of patients with subfoveal neovascular lesions secondary to AMD. Only one clinical trial (the TAP study) addressing the medical effectiveness of OPT with verteporfin was available for review. However, the TAP study is a large, well-designed, placebo-controlled, randomized trial which makes a substantial case in demonstrating that OPT with verteporfin is a medically reasonable and necessary treatment for a subset of patients with neovascular AMD. The trial’s investigators did a thorough job designing the protocols to protect against potential selection bias. The randomization process and masking procedures used were meticulous and successful, ensuring the validity of the study results. A sufficiently large study population was enrolled to ensure that the study had adequate power to detect true differences between treatment groups.
The primary and secondary health outcomes selected by the investigators were clinically and functionally appropriate measures of treatment benefit. The primary efficacy outcome (the percentage of patients who experience less than 15 letters lost) is a valid measure of the treatment’s effect on visual acuity. A loss of 15 letters or more in visual acuity is indicative of moderate visual loss.22 Secondary outcomes such as mean changes in contrast sensitivity and visual acuity, as well as the percentage of patients who experience a loss of 30 letters or more, generally correlate with observed differences in the primary efficacy outcome. These efficacy outcomes measure the treatment’s effect on visual functioning. In order to link improvements in visual acuity to actual physical improvements in the subfoveal CNV lesions, the TAP investigators also included fluorescein angiographic outcomes (such as lesion size, leakage, and progression). The results of these angiographic outcomes show a parallel between a lower risk of visual acuity loss and improvements in CNV lesions.
Overall, as indicated by Table 1, the verteporfin treatment group had significantly better outcomes in visual acuity and contrast sensitivity compared to the placebo group. The extent of vision loss was less in the verteporfin group compared to the placebo-treated group. For example, at 12 months, only 46.4% of the placebo group lost less than 15 letters of visual acuity compared to 61.2% of the verteporfin group. The results are durable over a two year period. At 24 months of follow-up, the differences in outcome between the two groups remain statistically significant. However, as demonstrated in Table 2, the treatment benefit observed in the verteporfin group appears to be limited to patients with predominantly classic subfoveal CNV lesions. The data suggests that the proportion of the lesion containing classic CNV may affect the extent of treatment benefit. Patients with 100% classic CNV lesions, a subset of the predominantly classic subgroup, attained the greatest benefit from verteporfin therapy. 76.6% of patients in the verteporfin treatment group with 100% classic CNV lost less than 15 letters in visual acuity at 12 months. This is compared to 67.3% for the entire predominantly classic subgroup and 61.2 % for the overall verteporfin group. As mentioned above, predominantly classic patients with some evidence of occult CNV still showed benefit from verteporfin therapy. Therefore, OPT with verteporfin will be covered for patients with subfoveal CNV lesions that are considered at least 50% classic.
Although TAP investigators used an acuity cut-off of 20/200 in their trial, HCFA does not believe that visual acuity should be a determinant of whether or not a patient is eligible for verteporfin treatment. This therapy neither reverses vision loss nor protects against additional vision loss. Verteporfin’s clinical effectiveness stems from its ability to slow the progressive loss of central vision due to neovascular AMD. In the TAP study, baseline visual acuity was similar for both groups (20/80-2). At 12 months, the mean visual acuity was 20/160+2 for the verteporfin group and 20/200 for the placebo group. The TAP study shows that verteporfin treatment has the ability to extend the amount of time the patient has between disease onset and blindness. One might think it reasonable to place a visual acuity cut-off at a point at which there is no salvageable visual function. However, this cut-off is highly subjective, depending on the individual patient. The determination of whether or not there is any visual function worth saving will be left to the patient’s treating physician.
There are some important outstanding issues that warrant attention. As mentioned above, no treatment data is available past 24 months. The data from the TAP study shows that patients in the verteporfin group were retreated an average of 5.6 treatments per patient (with a range of one to eight treatments) over a two-year period. Over 50% of these patients received six or more treatments during follow-up. It is uncertain whether the observed treatment benefit extends beyond two years. There are no indications as to the appropriate frequency of additional treatments or how many total treatments a patient will potentially need throughout the clinical management of their neovascular AMD. In addition, no criteria are given to help determine whether a patient has failed treatment or when treatment should be terminated prior to two years. These issues are important because, if the benefit of verteporfin therapy does not go beyond 24 months, there may be a chance that patients who continue to be treated for recurrent CNV leakage are being subjected to unnecessary treatment. The TAP study protocols, however, indicated that additional treatments were to be given if any CNV leakage was detected by a fluorescein angiogram taken at regular follow-up visits conducted every three months during the two-year investigational period of the trial. The demonstrated effectiveness of verteporfin is based on these protocols. It is not possible to establish a retreatment cutoff at the present time given the lack of data past 24 months. HCFA will therefore not limit the number of additional treatments. However, retreatment cases that exceed the average number of treatments observed in the TAP study will be subject to medical review. Given these areas of important scientific uncertainty regarding OPT, HCFA will continue to review the literature in this area and update its coverage policy as deemed appropriate.
Another outstanding issue that was not addressed in either TAP report is the subjective nature of interpreting fluorescein angiograms. Even among properly trained readers, there tends to be great variability in the interpretation of these tests. This disagreement among trained readers is particularly prominent in lesions that are approximately 50% classic. Discrepancies rarely occur when lesion composition is closer to either extreme (i.e. 0% classic or 100% classic). This is important because, as demonstrated by the TAP study, verteporfin is effective in a limited patient population. Fluorescein angiography is the only means through which this patient population can be identified. Given that this tool is not entirely objective, patients who may truly benefit from treatment may not receive it due to a misreading of the angiogram. Conversely, patients for whom treatment is inappropriate may unnecessarily receive treatment. Given the potential for reader variability, those administering verteporfin therapy will be subject to medical review at the discretion of the Medicare contractors, as they deem necessary, to ensure that treatment is being applied to the appropriate sub-population.
Based on the results of the TAP study and the above concerns, HCFA has decided to cover OPT with verteporfin for AMD patients with predominately classic subfoveal CNV lesions (where the area of classic CNV occupies ≥ 50% of the area of the entire lesion) as determined by a fluorescein angiogram. Other uses of OPT with verteporfin will not be covered. This includes the following patients:
- Patients with minimally classic CNV lesions (where the area of classic CNV occupies < 50% of the area of the entire lesion),
- Patients with juxtafoveal or extrafoveal CNV lesion (lesions outside the fovea),
- Patients who are unable to obtain a fluorescein angiogram,
- Patients with atrophic AMD.
At this time, HCFA will not cover OPT with verteporfin for any other indications due to a pending FDA review of an application submitted by QLT PhotoTherapeutics, Inc. (the makers of verteporfin) to expand the drug’s labeled indications to include other retinal disorders, such as pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, CNV due to certain retinal abnormalities, and idiopathic causes. HCFA is aware that clinical trials, such as the Verteporfin in Photodynamic Therapy (VIP) study, are investigating the effectiveness of this treatment in patients that develop CNV due to pathologic myopia. HCFA is interested in evaluating such evidence for these new indications once the FDA has completed its review.
1 www.nih.gov/news/WordonHealth/jan99/story01.htm.
2 Fine S, et al. 2000.
3 Hartnett M 1996.
4 Bressler S 1993.
5 Bressler N 1997.
6 Bressler N 2000.
7 Hartnett M 1996.
8 Bressler S 1993.
9 Bressler S 1993.
10 Bressler N 2000.
11 Bressler N 1993.
12 Bressler N 2000.
13 Arnold J 2000.
14 Bressler N 1993.
15 Fine S 2000.
16 Bressler N 1997.
17 Treatment of AMD with Photodynamic Therapy Study Group 2000.
18 Treatment of AMD with Photodynamic Therapy Study Group 2000.
19 Summaries for these meetings can be access at www.cms.hhs.gov/coverage/default.asp.
20 The second TAP report containing the results of the 24-month follow-up has been accepted for publication in the Archives of Ophthalmology.
21 Arnold J 2000.
22 Arnold J 2000.
Meeting Report
Meeting Report
Meeting Report


