TO: Administrative File: CAG-00439R
FROM: Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group
Joseph Chin, MD, MS
Deputy Director, Coverage and Analysis Group
JoAnna Baldwin, MS
Acting Director, Division of Policy and Evidence Review
Melissa Evans, PhD, MSAE
Acting Deputy Director, Division of Policy and Evidence Review
Kimberly Long
Lead Analyst
Carl Li, MD, MPH
Lead Medical Officer
Dhritiman V. Mukherjee, PhD, MS, MPH
Lead Epidemiologist
SUBJECT: Reconsideration— Final National Coverage Determination for Lung Cancer Screening with
Low Dose Computed Tomography (LDCT)
DATE: February 10, 2022
I. Decision
The Centers for Medicare & Medicaid Services (CMS) reconsidered the national coverage determination established at section 210.14 of the Medicare National Coverage Determinations manual and has determined that the evidence is sufficient to expand the eligibility criteria for Medicare beneficiaries receiving low dose computed tomography (LDCT) when the following criteria are met:
Beneficiary eligibility criteria:
- Age 50 – 77 years;
- Asymptomatic (no signs or symptoms of lung cancer);
- Tobacco smoking history of at least 20 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
- Current smoker or one who has quit smoking within the last 15 years; and
- Receive an order for lung cancer screening with LDCT.
Counseling and Shared Decision-Making Visit
Before the beneficiary’s first lung cancer LDCT screening, the beneficiary must receive a counseling and shared decision-making visit that meets all of the following criteria, and is appropriately documented in the beneficiary’s medical records:
- Determination of beneficiary eligibility;
- Shared decision-making, including the use of one or more decision aids;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment; and
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions.
Reading Radiologist Eligibility Criteria
For purposes of Medicare coverage of lung cancer screening with LDCT, the reading radiologist must have board certification or board eligibility with the American Board of Radiology or equivalent organization.
Radiology Imaging Facility Eligibility Criteria
For purposes of Medicare coverage, lung cancer screening with LDCT must be furnished in a radiology imaging facility that utilizes a standardized lung nodule identification, classification and reporting system.
The above policy simplifies requirements for the counseling and shared decision-making visit, removes the restriction that it must be furnished by a physician or non-physician practitioner, reduces the eligibility criteria for the reading radiologist, and reduces the radiology imaging facility eligibility criteria (including removes the requirement that facilities participate in a registry). See Appendix B for the expected manual language.
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology:
AAFP – American Academy of Family Physicians
ABR - American Board of Radiology
ACR – American College of Radiology
ACR/STR - American College of Radiology and Society of Thoracic Radiology
ACS – American Cancer Society
AHRQ - Agency for Healthcare Research and Quality
ALA – American Lung Association
APC - average annual percent change
ATS - American Thoracic Society
AWV – annual wellness visit
BRFSS – Behavioral Risk Factor Surveillance System
CAG – Coverage and Analysis Group
CAT – computerized axial tomography
CCI – Charlson comorbidity index
CHEST - American College of Chest Physicians
CI - confidence interval
CME - Continuing Medical Education
CMS - Centers for Medicare &Medicaid Services
COPD – chronic obstructive pulmonary disease
COSMOS - Continuous Observation of Smoking Subjects
CT – computed tomography
CTDIvol – computed tomography dose index
CXR – chest x-ray
DANTE – Detection and Screening of Early Lung Cancer by Novel Imaging Technology Molecular Assays
DLCST – Danish Lung Cancer Screening Trial
FDA – United States Food and Drug Administration
FDCA – Federal Food, Drug, and Cosmetic Act
GRADE - Grading of Recommendations Assessment, Development, and Evaluation
HIV – Human Immunodeficiency Virus
HR - hazard ratio
IQR - interquartile range
ITALUNG - Italian Lung Cancer Screening Trial
IDTF — Independent Diagnostic Testing Facility
IV - intravenous
JAMA – Journal of the American Medical Association
JNCCN – Journal of the National Comprehensive Cancer Network
KQ – key question
kVp - kilovoltage peak
LC – lung cancer
LD – low dose
LCSR - Lung Cancer Screening Registry
LCS - lung cancer screening
LDCT – low dose computed tomography
LSS - Lung Screening Study
Lung-RADS - Lung Imaging Reporting and Data System
LUSI - Lung Cancer Screening Intervention
mAs - milliampere-seconds
MCBS – Medicare Current Beneficiary Survey
MCLIR - maximum clinical incidence reduction
mGy – milligray
mSv – millisievert
MILD – Multicentric Italian Lung Detection
MOC - Maintenance of Certification
NCA - National Coverage Analysis
NCCN – National Comprehensive Cancer Network
NCD - National Coverage Determination
NCI – National Cancer Institute
NCRP – National Council on Radiation Protection and Measurements
NELSON - Nederlands–Leuvens Longkanker Screenings Onderzoek
NHIS - National Health Interview Survey
NIH – National Institutes of Health
NLST – National Lung Screening Trial
NPI - National Provider Identifier
NSCLC – non-small cell lung cancer
OR - odds ratio
PCPs – primary care providers or primary care physicians
PICO - population, intervention, comparator, and outcome
PLCO – Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial
PLHIV - persons living with HIV
PMA – premarket approval application
PPV – positive predictive value
RCT – randomized control trial
RR - rate ratio or risk ratio
RRL - relative radiation level
SCLC – small cell lung cancer
SEER – Surveillance, Epidemiology, and End Results
SES - socioeconomic status
SD – standard deviation
SDM – shared decision-making
SR - Society of Thoracic Radiology
T0 – time zero, baseline screening
UCLA – University of California at Los Angeles
UKLS - UK Lung Cancer Screening trial
US - United States
USPSTF – United States Preventive Services Task Force
WHO – World Health Organization
YR(S) – year(s)
YSQ - years since quitting
CMS initiated this national coverage determination (NCD) to reconsider coverage under the Medicare Program for lung cancer screening with low dose computed tomography (LDCT). The scope of our review is limited to the consideration of screening for lung cancer with low dose CT. Diagnostic CTs are
outside the scope of this national coverage analysis (NCA).
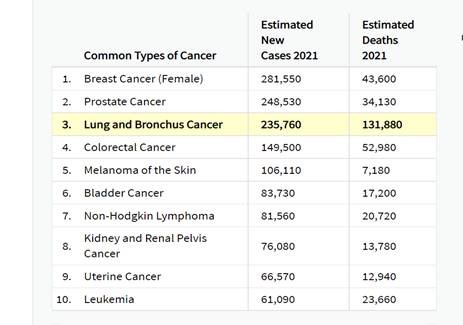
Lung cancer is the third most common cancer and the leading cause of cancer-related death in both men and women in the United States (NCI, 2021). It is an important issue for the Medicare population due to the age at diagnosis and age at death. In 2021, the National Cancer Institute (NCI) estimated that the number of new cases is over 235,000, with a median age at diagnosis of 71 years (NCI, 2021). Cancer of the lung and bronchus is estimated to account for over 130,000 deaths in 2021 (more than the total number of estimated deaths from colon, breast and prostate cancer combined) with a median age at death of 72 years (NCI, 2021).
(NCI, 2021)
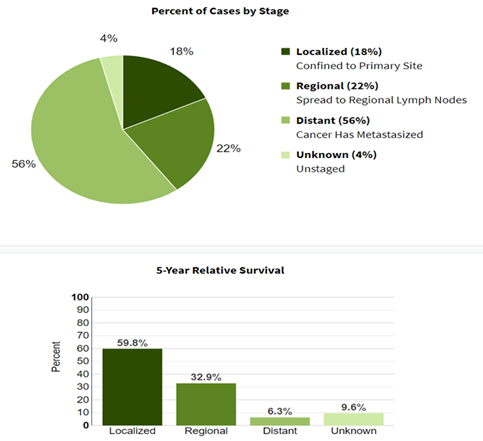
Overall mortality rates for lung and bronchus cancer have decreased over the past decade, averaging 3.8% each year over 2010–2019 (NCI, 2021). The majority of cases are still diagnosed at a late stage with a low five-year relative survival (NCI, 2021). Most patients diagnosed with lung cancer present with distant or metastatic disease; less than 20% are diagnosed with localized (i.e., stage 1) disease (NCI, 2021). Patients with localized disease have a 60% five-year survival rate, compared with 33% for those with regional spread to lymph nodes and 6% for those with distant pulmonary metastases (NCI,
2021).
Lung cancer has a generally poor prognosis, with overall observed five-year relative survival of 22.1% in 2013 (NCI, 2021). However, early-stage lung cancer has a better prognosis and is more amenable to treatment (USPSTF, Krist; 2021). By leading to earlier detection and treatment, screening for lung cancer can give patients a greater chance for cure (USPSTF, Krist; 2021).
(Stage and Survival for Lung Cancer; NCI, 2021)
Lung cancer is a proliferation of malignant cells arising in the tissues or airways of the lungs. Lung cancer has traditionally been classified into 2 major categories based on cell type and immunohistochemical and molecular characteristics: (1) non–small cell lung cancer (NSCLC), which collectively comprises adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (ACS, Lung Cancer; 2019), and (2) small cell lung cancer, which is more aggressive and has worse survival rates (Jonas, 2021; Travis, 2015; USPSTF, Krist; 2021). About 80% to 85% of lung cancers are non-small cell lung cancer (NSCLC) (ACS, Lung Cancer; 2019). These subtypes, which start from different types of lung cells are grouped together as NSCLC because their treatment and prognoses (outlook) are often similar. About 10% to 15% of all lung cancers are small cell lung cancer (SCLC) (ACS, Lung Cancer; 2019). This type of lung cancer tends to grow and spread faster than NSCLC. About 70% of people with SCLC will have cancer that has already spread at the time they are diagnosed (ACS, Lung Cancer; 2019). Screening is aimed at early detection of NSCLC rather than small cell lung cancer because the latter is much less common and typically spreads too quickly to be reliably detected at an early, potentially curable stage by screening (USPSTF, Krist; 2021).
The most important risk factor for lung cancer is smoking (Alberg, 2013). The risk of developing lung cancer is largely driven by age and smoking status, with smoking estimated to account for nearly 90% of all lung cancer cases (ACS, Risk Factors, 2019; Alberg, 2013; Siegel, 2021; USPSTF, Krist, 2021), with a relative risk of developing lung cancer approximately 20-fold higher in smokers than in nonsmokers (Samet, 1992). Just as important, older age is also associated with increasing incidence of lung cancer (USPSTF, Krist; 2021). Other risk factors for lung cancer include environmental exposures such as radon and asbestos, radiation therapy, other (noncancer) lung diseases, race/ethnicity, and family history (ACS, Risk Factors, 2019; Jonas, 2021). About 80% of lung cancer deaths are thought to result from smoking (ACS, Risk Factors; 2019).
Computed Tomography
The National Institutes of Health (NIH) National Cancer Institute (NCI) describes computed tomography (CT) as “an imaging procedure that uses special x-ray equipment to create detailed pictures, or scans, of areas inside the body. It is sometimes called computerized tomography or computerized axial tomography (CAT). . . . Each picture created during a CT procedure shows the organs, bones, and other tissues in a thin ‘slice’ of the body. The entire series of pictures produced in CT is like a loaf of sliced bread—you can look at each slice individually (2-dimensional pictures), or you can look at the whole loaf (a 3-dimensional picture). Computer programs are used to create both types of pictures. Modern CT machines take continuous pictures in a helical (or spiral) fashion rather than taking a series of pictures of individual slices of the body, as the original CT machines did. Helical CT (also called spiral CT) has several advantages over older CT techniques: it is faster, produces better quality 3-D pictures of areas inside the body, and may detect small abnormalities better” (NIH, NCI; 2019).
United States Preventive Services Task Force (USPSTF)
In 2021, the most recent recommendation was released to update its 2013 recommendation: “The USPSTF recommends annual screening for lung cancer with LDCT in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (USPSTF, Krist; 2021).
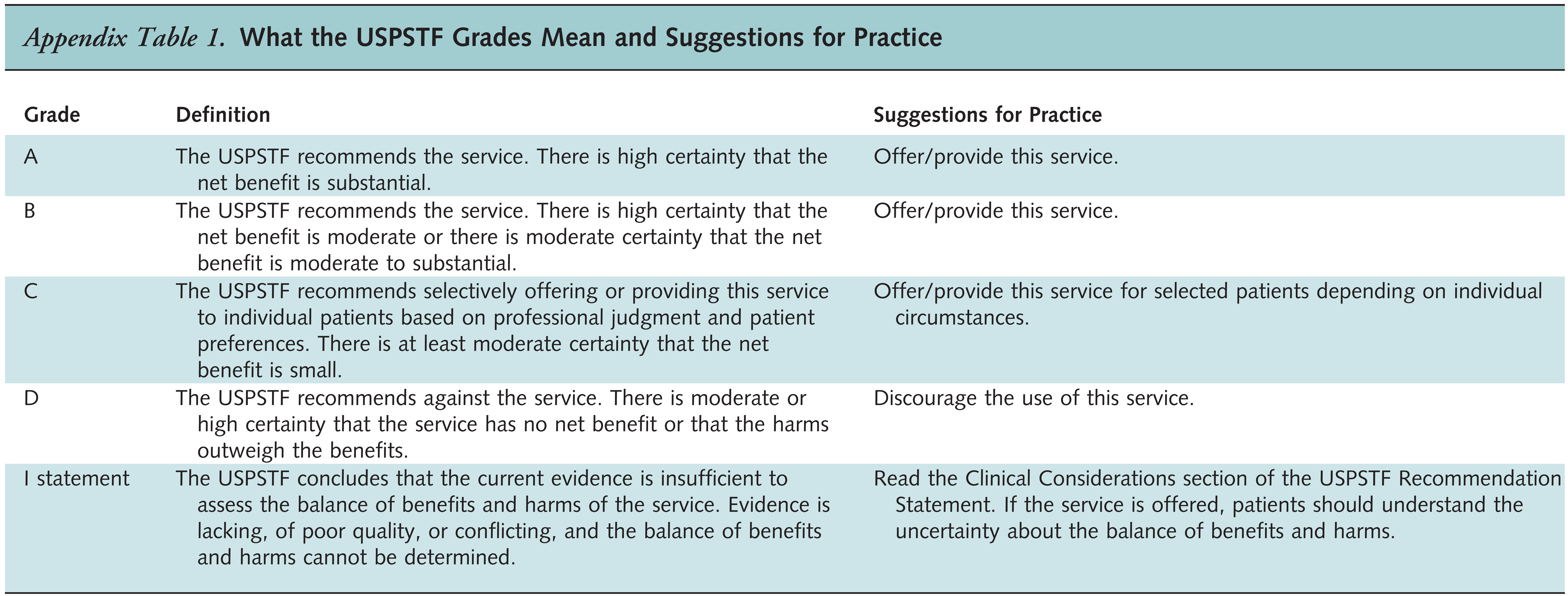
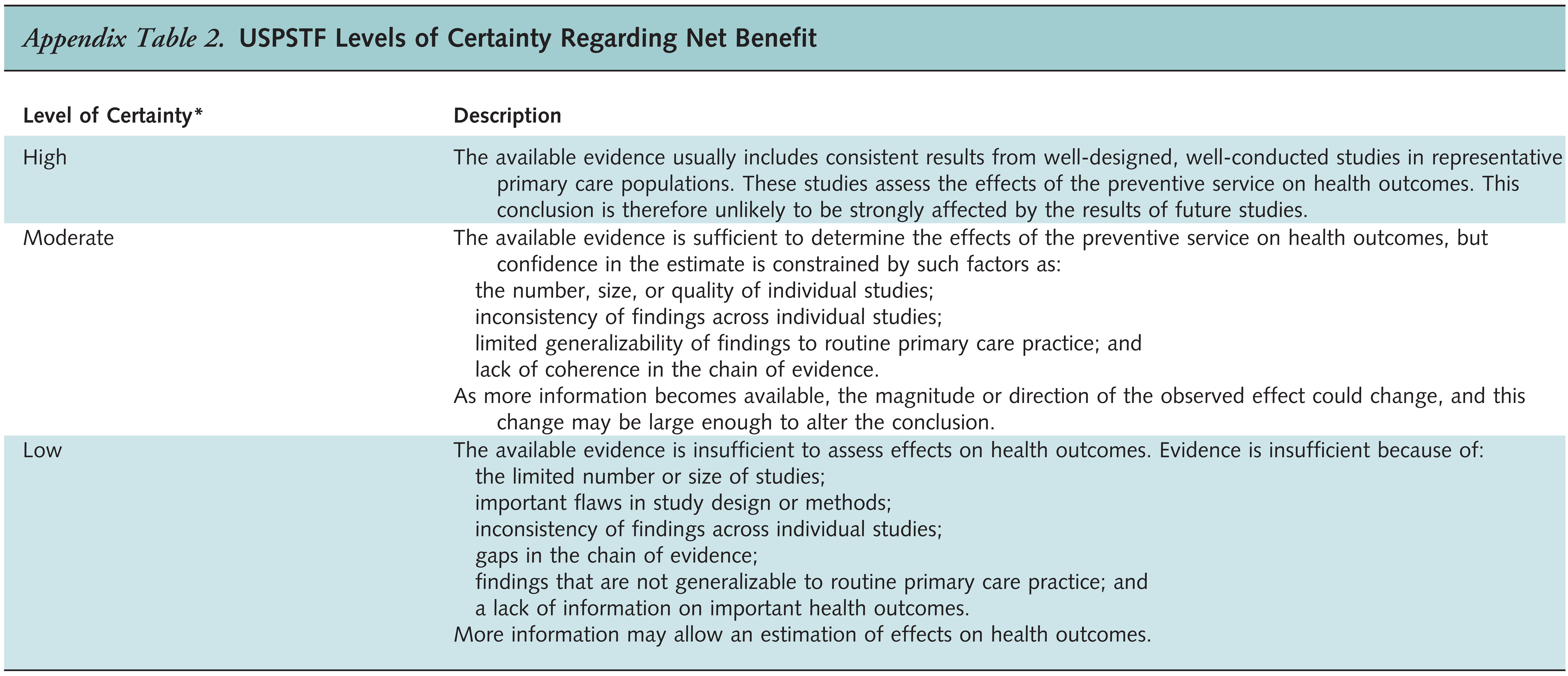
The USPSTF assigns one of five letter grades to each of its recommendations (A, B, C, D, I). The following tables from Moyer et al. (2014) on behalf of the USPSTF provide the current grade definitions and descriptions of levels of certainty after July 2012.
(Moyer, 2014)
(Moyer, 2014)
III. History of Medicare Coverage
Pursuant to §1861(ddd)(1) of the Act, the Secretary may add coverage of "additional preventive services" if certain statutory requirements are met. Specifically, the definition of ‘additional preventive services’ permits coverage under Medicare Part B for certain services that were not already included as “preventive services” and if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Services Task Force.
(3) Appropriate for individuals entitled to benefits under Part A or enrolled under Part B.
We have established regulations implementing this statute that are codified at 42 CFR § 410.64.
CMS issued an NCD on February 5, 2015 establishing coverage for lung cancer screening with LDCT. The NCD includes beneficiary eligibility criteria, a counseling and shared decision-making visit, reading radiologist eligibility criteria, and radiology imaging facility eligibility criteria.
The policy is codified in section 210.14 of the Medicare National Coverage Determinations (NCD) Manual (Pub. 100-03). Section 210.14 of the NCD Manual is included in Appendix C.
A. Current Request
CMS received a complete, formal joint request to reconsider the Lung Cancer Screening with Low Dose Computed Tomography (LDCT) NCD from the GO2 Foundation for Lung Cancer, The Society of Thoracic Surgeons, and American College of Radiology® (ACR®).
The request letter is available at https://www.cms.gov/medicare-coverage-database/view/ncacal-tracking-sheet.aspx?ncaid=304
B. Benefit Category
Medicare is a defined benefit program. For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Act. CMS is authorized to cover "additional preventive services" if certain statutory requirements are met as provided under §1861(ddd) of the Social Security Act.
IV. Timeline of Recent Activities
| Date | Actions Taken |
|---|
| May 18, 2021 |
CMS initiates this national coverage analysis. A 30-day public comment period begins.
|
| June 17, 2021 |
First public comment period ends. CMS receives 170 comments. |
| November 17, 2021 |
Proposed Decision Memorandum posted. 30-day public comment period begins. |
| December 17, 2021 |
Second public comment period ends. CMS receives 49 public comments. |
V. Food and Drug Administration (FDA) Status
CT imaging systems and post-processing software go through the 510(k) process at the FDA to obtain clearance for commercial distribution. To obtain 510(k) clearance, the sponsor must demonstrate that the device is substantially equivalent in terms of its intended use, technological characteristics, and safety and effectiveness to CT systems previously cleared or on the market prior to the 1976 Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (FDCA) that do not require approval of a premarket approval application (PMA). FDA has authorized CT systems with indications for low-dose lung cancer screening.
Counseling services are not generally under the purview of the FDA.
VI. General Methodological Principles
When making national coverage determinations concerning additional preventive services, CMS applies the statutory criteria in § 1861(ddd) of the Social Security Act and regulations at 42 CFR 410.64, and evaluates relevant clinical evidence to determine whether or not the service is reasonable and necessary for the prevention or early detection of illness or disability, is recommended with a grade of A or B by the USPSTF, and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B of the Medicare program.
VII. Evidence
The evidence summarized in this section includes the peer-reviewed, published clinical research pertinent to lung cancer screening with LDCT. Our assessment focuses on the key evidence questions below.
1. Evidence Questions
Question 1: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is recommended with a grade of A or B by the United States Preventive Services Task Force?
Question 2: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is reasonable and necessary for the prevention or early detection of illness or disability?
Question 3: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is appropriate for Medicare beneficiaries?
2. External Technology Assessments
CMS did not request an external technology assessment (TA) on this issue.
3. Internal Technology Assessment
Literature Search Methods
CMS searched PubMed for publications from the period of August 2014 to July 2021. We chose the starting search date as August 2014 since the literature search for the 2015 National Coverage Determination’s internal technology assessment on screening for lung cancer with low dose CT ended in August 2014. General keywords included low dose computed tomography, screening, mortality, and lung cancer. Publications that presented original human clinical data on screening with low dose CT for lung cancer were considered. Keywords for the search included “low dose computed tomography”, “low dose CT”, “LDCT”, “screening”, “survival”, “mortality”, “death”, and “lung cancer”. CMS staff searched the bibliographies of relevant publications for other pertinent studies. Abstracts, meeting presentations, reviews, animal studies, publications on emphysema and asbestos, cost analyses, microsimulation modeling studies, mechanistic biomarker studies, and non-English language publications were excluded. Studies with fewer than 10 cases and those not involving human subjects were excluded. The reviewed evidence was gathered from articles submitted by the requester, and from the public comments and the PubMed literature search.
Articles were included if they conformed to the following eligibility criteria:
Inclusion criteria:
- Studies using low dose computed tomography (low dose CT) for lung cancer screening
- Articles published in scientific journals
- Technology assessments, guidelines, consensus statements, or meta-analyses
- English language
- Adult
- Human
- Literature from United States, Canada, the United Kingdom, Europe, Australia, China and other Southeast Asian countries
Exclusion criteria:
- Non-English language articles
- Technical, nonclinical articles (e.g., animal, biomechanical, cadaver)
- Conference abstracts, meeting presentations
- Studies conducted outside the countries listed above
- Studies published before 2014
- Studies with less than 10 participants
- Studies not related to low dose computed tomography
- Studies not related to lung cancer
| Table 1. Age Eligibility Criteria for Low Dose CT Randomized Controlled Trials |
| First Author (year published), Study Acronym, Study Design, Population: Country, Overall Sample Size (n) |
Age Inclusion Criteria (years) |
Age Distribution in Screening Study Population (mean, lowest and highest age groups, frequency; n, years) |
Race (percent) |
Women (percent) |
Timing: Follow Up Duration (mean; years) |
Outcome: Lung Cancer Mortality: Low Dose CT Arm Compared Control Arm (HR, RR, or OR, 95% CI; p value) |
| Becker (2020) LUSI (RCT) Germany (4,052) |
50 to 69 |
Median: 55
50-54: 46.4% (942/2029)
65-69: 11.1% (225/2029) |
Not shown |
35.3% |
8.8 yr (median) |
HR: 0.74 (0.46 –1.19; p = 0.21)
HR women: 0.31 (0.10 –0.96, p = 0.04) |
| de Koning (2020) NELSON (RCT) Netherlands, Belgium (15,792 total; 13,195 men, 2594 women) |
50 to 74 |
Median: males: 58 (IQR: 55-63)
Range 46-76
50-54: 24.6% (1611/6560) ≥ 75: 0.6% (40/6560) |
Not shown |
16.4% |
10 yr (actual) |
RR: 0.76 (0.61 - 0.94; P = 0.01)
RR men 50-54: 0.85 (0.48 - 1.50) |
| Doroudi (2018) LSS (RCT) United States (3,318) |
55 to 74 |
No age data for study population shown |
Not shown |
Not shown |
5.2 yr |
RR: 1.24 (0.74 - 2.08) |
| Field (May, 2016) UKLS (RCT) United Kingdom (4,055) |
50 to 75 |
50-55: 0.7%
71-75: 22.4% |
99% White
1% non-white |
25.1% |
Not shown |
No lung cancer mortality; Only lung cancer incidence |
| Field (February, 2016) UKLS (RCT) United Kingdom (4,055) |
50 to 75 |
Mean: 67.1 (SD 4.1)
Median: 67 Range: 50 to 75 |
99% White
1% non-white |
25.1% |
1 yr |
Lung cancer mortality not assessed; Confirmed lung cancer |
| Infante (2015) DANTE (RCT) Italy (2,450 men) |
60 to 74 |
Mean (95% CI): 64.6 (64.3–64.8)
Median: 64.0 (IQR 5) |
Not shown |
0% |
8.35 yr (median) |
HR: 0.993 (0.69–1.43) |
| Infante (Pooled, 2017) (pooled analysis DANTE and MILD) Italy (6,549) |
≥ 49 to 74 |
Median: 61 (IQR 10)
≤ 60: 48.5% (1766/3640)
>60: 51.5% (1874/3640) |
Not shown |
21.5% |
8.2 yr (median) |
Pooled HR: 0.83 (0.61–1.12) |
| National Lung Screening Trial Research Team (2019) NLST (RCT) United States (53,452) |
55 to 74 |
55-74: 42.8% (11442 / 26722)
70-74: 8.8% (2354/26722) |
89.6% White
4.4% Black
1.7% Hispanic
2.0% Asian
0.3% American
Indian / Native Alaskan
0.3% Native
Hawaiian /Pacific Islander
1.6% Other /
unknown |
41.0% |
12.3 yr (median) |
RR dilution-adjusted: 0.89 (0.80–0.997) |
| Tanner (2016) NLST (secondary analysis of NLST) United States (53,452) |
55 to 74 |
Not shown |
89.6% White
4.4% Black
6.0% Other / Missing |
41.0% |
Not shown |
HR: 0.62 (0.51–0.76) |
| Paci (2017) ITALUNG (RCT) Italy (3,206) |
55 to 69 |
Mean: 60.9
<55: 3% (53/1613)
>69: 0.3% (5/1613) |
Not shown |
35.3% |
9.3 yr |
RR: 0.70 (0.47 - 1.03; p = 0.07) |
| Paci (2021) ITALUNG (secondary analysis) Italy (3,206) |
55 to 69 |
<55: 3%
≥ 70: 0% |
Not shown |
35% |
10 yr |
10 yr survival rates for lung cancer cases: 64% active arm vs 60% control arm (p = 0.69) |
| Pastorino, Silva (Prolonged; July, 2019) MILD (RCT) Italy (4,099) |
49 to 75 |
Median: 58
< 55: 32.5% (773/2376)
≥ 70: 3.8% (90/2376) |
Not shown |
33.7% |
10 yr |
HR: 0.61 (0.39 – 0.95; p = 0.02) |
| Pastorino, Sverzellati (Ten Year; September, 2019) MILD (secondary analysis of RCT) Italy
(2,376) (subset of screening arm) |
49 to 75 |
<55: 32.5% (773/2376)
≥ 65: 15.5% (368/2376) |
Not shown |
31.6% |
10 yr |
Biennial (low dose CT every 24 months) arm compared to annual arm: HR: 1.10 (0.59 -2.05) |
| Rampinelli (2017) COSMOS (secondary retrospective analysis of observational screening trial) Italy (5,203) |
50 and older |
50-54: 33.8% (1759/5203)
≥ 65: 12% (658/5203)
age range not shown |
Not shown |
33.9% |
10 yr |
No lung cancer mortality, but lifetime attributable risk of cancer incidence and radiation exposure dose |
| Wille (2016) DLCST (RCT) Denmark (4,104) |
50 to 70 |
Mean 57.9 ± 4.8 |
Not shown |
44.8% |
> 5 yrs after last screen, mean follow up duration not shown |
HR: 1.03 (0.66 –1.6; p = 0.89) |
| Table 2. Age Eligibility Criteria for Low Dose CT Observational Studies and Meta-Analyses |
| First Author, (year published) Study Acronym, Study Design |
Population: Country Overall Sample Size (n) |
Age Inclusion Criteria (years) |
Age Distribution in Screening Study Population (mean; lowest and highest age groups; frequency;
n, years) |
Timing: Follow up Duration (mean, median; years) |
Outcome: Lung Cancer Mortality: Low Dose CT Arm Compared Control Arm (HR, RR, or OR, 95% CI; p value) |
| Dement (2020) (prediction modeling) |
United States (17,069) |
Not shown |
No age data |
Not shown |
Modeling results |
| Horeweg (2014) NELSON (RCT) |
Netherlands, Belgium (7,155) |
50 to 75 |
Median: 58.0 (IQR 54.0-62.0) |
Not shown |
No lung cancer mortality; Results: sensitivity, specificity, positive predictive value; interval cancers, screen-detected lung cancer |
| Leleu (2020) DEP KP80 (single arm prospective study) |
France (1,307) |
55 to 74 |
Mean: 61.8 |
Not shown |
No lung cancer mortality; Results: prevalence of lung cancer 2.7%. |
| Liang (2019) (cancer registry study) |
Shanghai and two districts, China (over 9.5 million) |
Inclusion criteria for age not shown |
35-39
40-44
45-49
50-54
70-74
75-79
80-84
≥85
no sample sizes shown |
No follow up period noted |
Lung cancer mortality rate as average annual percent change [APC] = -1.71%, 95% CI: -2.98% –0.04%, P= 0.015) |
| Nawa (2019) (retrospective cohort study) |
Japan (210) |
≥ 50 |
Not shown |
4 to 8 years after introduction of CT screening |
Reduction (24%) in lung cancer mortality |
| Patz (2016) NLST (retrospective cohort subgroup analysis) |
United States (26,231, with negative T0 screen) |
55 to 74 |
Negative T0 screen
60 (55-74) |
6.4 yr |
Negative T0 screen vs all T0 screen: 185.8 (95% CI 162.2–211.9) per 100,000 person-years vs 277.2 (252.3–303.9) |
| Rulli (2020) (retrospective review) |
United States (2,924) |
55 to 79 |
No age specific data shown |
3 yr |
Lung cancer mortality rate 239 per 100,000 patients. |
| White (2020) (retrospective review) |
United States (962) |
Not shown |
No age reported |
Not shown |
Lung cancer rate |
| Whittaker Brown (2019) NLST (subgroup analysis of interstitial lung abnormalities) |
United States (50,206) |
55 to 74 |
Median 61 (IQR 58-66) |
Not shown |
HR: 1.51 (1.13-2.03) |
| Wu (2019) (retrospective cohort study) |
Asian population (2,883) |
Not shown |
59.91 ± 8.14 |
Not shown |
Overall mortality of 10.75% in screened group |
| Meta-Analyses |
| Huang (2019) (9 RCTs) |
Italy, Denmark, Germany, US, Netherlands, Belgium, China (97,244) |
45-60 to 69-75 |
Mean 57.9 -61.0 ± 4.8-5.8
Median 57.0-64.0 (IQR 5-8)
Range 55-74 |
Heterogeneous |
Pooled RR: 0.83 (0.76–0.90, I2 = 1%) |
| Sadate (2020) (7 RCTs) |
Italy, Denmark, Germany, United States, Belgium, The Netherlands (84,558) |
≥ 49-60 to 69-74 |
Age data not shown for study population |
3-10 yr |
Pooled RR: 0.83 (0.76-0.91; I2 = 0%) |
| Wang (2018) (4 RCTs) |
United States, Italy (2), Denmark, (64,129) |
≥ 49-60 to 70-74 |
Age data not shown for study population |
Median 33.7 months to 6.5 yr; 4.8 person-years |
Pooled OR: 1.13 (0.78–1.64; I2 > 75%) |
| Yang (2019) (4 RCTs) |
United States, Italy (2), Denmark (64,468) |
>49-60 to 70-74 |
Age data not shown for study population |
Heterogeneous: 6 yr 3.5 months to 9.47 yr |
Pooled RR: 0.94 (0.74-1.19; p = 0.62; I2 = 43.3%) |
| Table 3. Smoking History Eligibility Criteria for Low Dose CT Randomized Controlled Trials and Meta-Analyses |
| First Author, (year published) Study Acronym, Study Design |
Smoking History Inclusion Criteria (pack-years) |
Smoking History Study Population Distribution in Intervention Arm (mean ± SD; frequency, range, or percent; pack-years) |
Smoking Cessation Inclusion Criteria (years since quitting smoking) |
Smoking Cessation Duration Since Quitting: Distribution in Study Population Intervention Arm
(n, years since quitting) |
Timing: Follow up Duration (mean, median; years) |
Outcome: Lung Cancer Mortality: Low Dose CT Arm Compared Control Arm (HR, RR, or OR, 95% CI; p value) |
| Becker (2020) LUSI (RCT) |
At least 25 years smoking of at least 15 cigarettes per day, or at least 30 years smoking of at least 10 cigarettes per day |
No pack-years shown |
Stopped smoking not more than 10 years before invitation to screening |
None shown |
8.8 yr (median) |
HR: 0.74 (0.46 – 1.19; p = 0.21) HR women: 0.31 (0.10 –0.96, p = 0.04) |
| de Koning (2020) NELSON (RCT) |
>15 cigarettes a day for > 25 years or >10 cigarettes a day for >30 years |
Median pack-year: 38.0 Interquartile range: 29.7 – 49.5 Range of pack-years: 0.4 to 159.5 |
Quit ≤ 10 years ago |
1.7% (49/2,908) quit smoking history of > 10 years since cessation of smoking |
10 yr (actual) |
RR: 0.76 (0.61 - 0.94; P = 0.01)
RR men 50-54: 0.85 (0.48 - 1.50) |
| Doroudi (2018) LSS (RCT) |
30 pack-year history of cigarette smoking |
None shown |
Quit within the last 10 years |
None shown |
5.2 yr |
RR: 1.24 (0.74 - 2.08) |
| Field (May, 2016) UKLS (RCT) |
None shown |
93.4% smoking duration of 20+ years,
5.8% smoking duration of 10-19 years |
None shown |
None shown |
None shown |
No lung cancer mortality Results: only lung cancer incidence |
| Infante (2015) DANTE (RCT) |
20+ pack-years |
Mean pack-years: 47.3 (95% CI 45.7 - 49.0), standard error of mean: 0.8
Median pack-years: 45.0 (IQR 28.5) |
Had quit less than 10 years before recruit-ment |
None shown |
8.35 yr (median) |
HR: 0.993 (0.688–1.433) |
| Infante (Pooled, 2017) (pooled analysis DANTE and MILD) |
None shown |
None shown |
None shown |
None shown |
8.2 yr (median) |
Pooled HR: 0.83 (0.61–1.12) |
| National Lung Screening Trial Research Team (2019) NLST (RCT) |
Minimum of 30 pack-years of cigarette smoking |
Median pack-years = 48 (25th/75th percentile: 39/66) |
Had quit within the past 15 years |
None shown |
12.3 yr (median) |
RR dilution-adjusted: 0.89 (0.80–0.997) |
| Tanner (2016) NLST (secondary analysis of NLST) |
None shown |
None shown |
None shown |
None shown |
None shown |
HR, 0.62 (0.51 – 0.76). |
| Paci (2017) ITALUNG (RCT) |
At least 20 pack-years in last 10 years |
Median pack-years of smoking: 40 pack-years |
Quit more than 10 years ago excluded |
None shown |
9.3 yr |
RR: 0.70 (0.47 to 1.03; p = 0.07) |
| Paci (2021) ITALUNG (secondary analysis) |
None shown |
None shown |
None shown |
None shown |
10 yr |
10 yr survival rates for lung cancer cases: 64% active arm vs 60% control arm (p = 0.689) |
| Pastorino, Silva, (Prolonged, July, 2019) MILD (RCT) |
≥ 20 pack- years |
< 30 pack-years of cigarette smoking: 21.9% (521/2,376); Median pack-years: 39 pack-years of smoking |
Former smoker from < 10 years ago |
None shown |
10 yr |
HR: 0.61 (0.39 – 0.95; p =0.02) |
| Pastorino, Sverzellati (Ten Year, September 2019) MILD (secondary analysis of screening arm in RCT) |
None shown |
None shown |
None shown |
None shown |
10 yr |
Biennial (low dose CT every 24 months) arm compared to annual arm: HR: 1.10 (0.59-2.05) |
| Rampinelli (2017) COSMOS (secondary analysis of an observational screening trial) |
≥ 20 pack-years of smoking |
None shown |
None shown |
None shown |
10 yr |
No lung cancer mortality, Results: lifetime attributable risk of cancer incidence and radiation exposure dose |
| Wille (2016) DLCST (RCT) |
Minimum of 20 pack-years of smoking |
Mean (SD) pack-years: 36.4 ± 13.4 pack-years |
Have quit after the age of 50 years and within the previous 10 years |
None shown |
> 5 yrs after last screen, mean follow up duration not shown |
HR: 1.03 (0.66–1.6; p = 0.888) |
| Meta-Analyses |
| Huang (2019) (9 RCTs) |
> 20 pack-years to > 30 pack-years, and included > 15 cigarettes a day for > 25 years or > 10 cigarettes per day for > 30 years |
Mean ± SD: 36.4 ± 13.4,
Median (IQR) ranged from 36 (IQR not reported) to 38.0 (19.8) to 45.0 (28.5) to 48 (27) to 54 (IQR not reported) |
From < 10 years since quitting to < 15 years since quitting |
None shown |
Hetero-geneous |
RR: 0.83 (0.76–0.90, I2 = 1%) |
| Sadate (2020) (7 RCTs) |
Average smoking history greater than 15 pack-years |
Median pack-years: 39
Mean (95% CI) ranged from 36.4 (95% CI 23 – 49.8) to 38.0 (95% CI 29.7 – 49.5) to 47.3 (95% CI 45.7 – 49) to 56.04 (no 95% CI reported) |
None shown |
None shown |
3-10 yr |
RR: 0.83 (0.76 - 0.91; I2 = 0%) |
| Wang (2018) (4 RCTs) |
Minimum of 20 pack-years to more than 30 pack-years |
None shown |
Quitting within 10 years of study enrollment to less than 15 years since quitting to quitting after age 50 years and within 10 years before trial enrollment |
None shown |
Median 33.7 months to 6.5 yr; 4.8 person-years |
OR: 1.13 (0.78–1.64; I2 > 75%) |
| Yang (2019) (4 RCTs) |
None shown |
None shown |
None shown |
None shown |
Hetero-geneous: 6 yr 3.5 months to 9.47 yr |
Pooled RR: 0.94 (0.74-1.19; p = 0.62; I2 = 43.3%) |
RCT - randomized controlled trial
COSMOS - Continuous Observation of Smoking Subjects
DANTE - Detection And screening of early lung cancer with Novel imaging Technology and molecular assays
DLCST - Danish Lung Cancer Screening Trial
ITALUNG - Italian Lung Cancer Screening Trial
LSS - Lung Screening Study
LUSI - Lung cancer Screening Intervention
MILD - Multicentric Italian Lung Detection
NELSON - Nederlands–Leuvens Longkanker Screenings Onderzoek
NLST - National Lung Screening Trial
UKLS - UK Lung Cancer Screening trial
HR - hazard ratio, RR - rate ratio or risk ratio, OR - odds ratio, CI - confidence interval, SD – standard deviation, IQR - interquartile range, T0 – baseline screening, yr(s) – year(s)
Evidence-based Guidelines
US Preventive Services Task Force, Krist AH, Davidson KW, Mangione CM, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021 Mar 9;325(10):962-970. doi: 10.1001/jama.2021.1117. PMID: 33687470
Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021 Mar 9;325(10):971-987. doi: 10.1001/jama.2021.0377. PMID: 33687468
Moyer VA; U.S. Preventive Services Task Force (USPSTF). Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014 Mar 4;160(5):330-8. doi: 10.7326/M13-2771. PMID: 24378917
2021 USPSTF recommendation statement (USPSTF, Krist; 2021)
“The USPSTF recommends annual screening for lung cancer with LDCT in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (USPSTF, Krist; 2021). “This recommendation replaces the 2013 USPSTF statement that recommended annual screening for lung cancer with LDCT in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years” (USPSTF, Krist; 2021).
“The USPSTF concludes with moderate certainty that annual screening for lung cancer with LDCT has a moderate net benefit in persons at high risk of lung cancer based on age, total cumulative exposure to tobacco smoke, and years since quitting smoking. The moderate net benefit of screening depends on limiting screening to persons at high risk, the accuracy of image interpretation being similar to or better than that found in clinical trials, and the resolution of most false-positive results with serial imaging rather than invasive procedures.” (USPSTF, Krist; 2021).
2013 USPSTF recommendation statement (Moyer, USPSTF; 2014)
In 2013, the US Preventive Services Task Force (USPSTF) recommended annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 55 to 80 years who have a 30–pack-year smoking history and currently smoke or have quit within the past 15 years (B recommendation). The USPSTF recommended that screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery (Moyer, USPSTF; 2014).
“The USPSTF concludes with moderate certainty that annual screening for lung cancer with LDCT is of moderate net benefit in asymptomatic persons who are at high risk for lung cancer based on age, total cumulative exposure to tobacco smoke, and years since quitting smoking” (Moyer, USPSTF; 2014).
Donnelly EF, Kazerooni EA, Lee E, et al., and Expert Panel on Thoracic Imaging: ACR Appropriateness Criteria Lung Cancer Screening. J Am Coll Radiol. 2018 Nov;15(11S):S341-S346. doi: 10.1016/j.jacr.2018.09.025. PMID: 30392603
In 2018, an expert panel on thoracic imaging assembled by the American College of Radiology reported that “the American College of Radiology Appropriateness Criteria are evidence-based guidelines for specific clinical conditions that are reviewed annually by a multidisciplinary expert panel. The guideline development and revision include an extensive analysis of current medical literature from peer reviewed journals and the application of well-established methodologies (RAND/UCLA Appropriateness Method and Grading of Recommendations Assessment, Development, and Evaluation or GRADE) to rate the appropriateness of imaging and treatment procedures for specific clinical scenarios. In those instances where evidence is lacking or equivocal, expert opinion may supplement the available evidence to recommend imaging or treatment” (Donnelly, 2018).
In 2018, the American College of Radiology expert panel on thoracic imaging summarized their findings by reporting that “for patients between the age of 55 and 80 with 30 or more pack-years smoking history who currently smoke or who have quit within the last 15 years should undergo lung cancer screening with low-dose CT. In patients who do not meet these criteria but who have additional risk factors for lung cancer, lung cancer screening with low-dose CT is controversial but may be appropriate. Imaging is not recommended for lung cancer screening of patient younger than 50 years of age or patients older than 80 years of age or patients of any age with less than 20 packs per year history of smoking and no additional risk factor (i.e., radon exposure, occupational exposure, cancer history, family history of lung cancer, history of [chronic obstructive pulmonary disease] (COPD), or history of pulmonary fibrosis” (Donnelly, 2018).
Radiation Dosing
As a special imaging consideration, the American College of Radiology Appropriateness Criteria expert panel stated that “in general, acceptable low-dose lung cancer screening CT scans should be performed according to the guidelines in the ACR–STR [American College of Radiology (ACR)-Society of Thoracic Radiology (STR)] Practice Parameter for the Performance and Reporting of Lung Cancer Screening Thoracic Computed Tomography (CT)” (Donnelly, 2018). Additionally, “because there is a wide range of radiation exposures associated with different diagnostic procedures, a relative radiation level (RRL) indication has been included for each imaging examination. The RRLs are based on effective dose, which is a radiation dose quantity that is used to estimate population total radiation risk associated with an imaging procedure” (Donnelly, 2018).
Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report – Executive Summary, CHEST (2021), doi: https://doi.org/10.1016/j.chest.2021.07.003.
Mazzone PJ, Silvestri GA, Souter LH, et all. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report, CHEST (2021), doi: https://doi.org/10.1016/j.chest.2021.06.063.
“The purpose of this guideline is to update the evidence base for the benefit, harms, and implementation of low-dose chest CT (LDCT) screening. The American College of Chest Physicians (CHEST) used the updated evidence base to provide recommendations where the evidence allows, and statements based on experience and expert consensus where it does not. Approved panelists reviewed previously developed key questions using the PICO (population, intervention, comparator, and outcome) format to address the benefit and harms of low-dose CT screening, as well as key areas of program implementation. A systematic literature review was conducted using MEDLINE via PubMed, Embase, and the Cochrane Library on a quarterly basis since the time of the previous guideline publication. The systematic literature review identified 75 additional studies that informed the response to the 12 key questions that were developed. . . . Evidence suggests that low-dose CT screening for lung cancer can result in a favorable balance of benefit and harms. The selection of screen-eligible individuals, the quality of imaging and image interpretation, the management of screen detected findings, and the effectiveness of smoking cessation interventions, can impact this balance.” (Mazzone, Executive Summary; 2021).
Recommendations
The panel drafted and graded recommendations based on the results of the meta-analyses and evidence profiles. Recommendations were graded according to CHEST’s grading system which uses the GRADE approach. (Mazzone, Panel Report 2021). The recommendations were either “strong” or “weak” according to this approach. Strong recommendations use the wording “we recommend” and weak recommendations use the wording “we suggest” (Mazzone, Panel Report 2021).
Excerpt from Recommendations (Mazzone, Panel Report 2021)
1. For asymptomatic individuals age 55 to 77 who have smoked 30 pack-years or more and either continue to smoke or have quit within the past 1 years, we recommend that annual screening with low dose CT should be offered. (Strong recommendation, moderate quality evidence) 2. For asymptomatic individuals who do not meet the smoking and/or age criteria in Recommendation #1, are age 50-80, have smoked 20 pack-year or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with low dose CT should be offered. (Weak recommendation, moderate quality evidence) 4. For individuals who have accumulated fewer than 20 pack-years of smoking or are younger than age 50 or older than 80, or have quit smoking more than 15 years ago, and are not projected to have a high net benefit from lung cancer screening based on clinical risk prediction or life-year gained calculators, we recommend that low dose CT screening should not be performed. (Strong recommendation, moderate quality evidence)
NCCN (National Comprehensive Cancer Network). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Lung Cancer Screening, Version 1.2021 — December 17, 2020. https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed 6/17/2021.
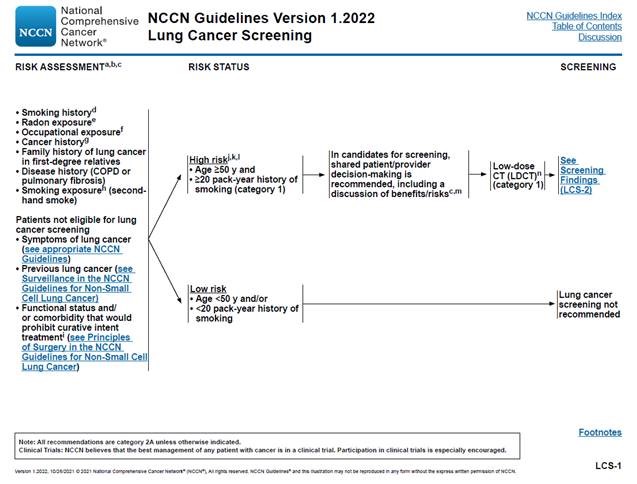
In the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Lung Cancer Screening Version 1.2021, the NCCN Lung Cancer Screening Panel defined the risk status of “high risk” as “age ≥ 50 years and ≥ 20 pack-year history of smoking” with these high-risk persons candidates for low dose CT (LDCT) screening (category 1) where “shared patient/physician decision-making is recommended, including a discussion of benefits/risks” (NCCN, 2020). For NCCN categories of evidence and consensus, Category 1 is defined as “based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate” (NCCN, 2020). The updates in the clinical pathway algorithms in the current Version 1.2021 (NCCN, 2020) of the NCCN Guidelines for Lung Cancer Screening from Version 1.2020 include high risk status being modified with the age range from 55 to 77 years to ≥ 50 years and from ≥ 30 to ≥ 20 pack-year history of smoking (NCCN, 2020).
Radiation Dose
The guidelines report that “all screening and follow-up chest CT scans should be performed at low-dose (100-120 kVp [kilovoltage peak] and 40-60 mAs [milliampere-seconds] or less), unless evaluating mediastinal abnormalities or lymph nodes, where standard-dose CT with IV [intravenous] contrast might be appropriate” (NCCN, 2020).
There is a section in the NCCN guidelines on low-dose computed tomography (LDCT) acquisition, storage, interpretation, and nodule reporting (Lung-RADS) which describes radiation exposure based on the patient’s body mass index or BMI (NCCN, 2020). This section further describes the acquisition parameters for LDCT, such as slice width and slice interval, and how to report nodule parameters, such as size and density of the nodule found on LDCT (NCCN, 2020). Additionally, the NCCN guidelines make clear that “for lung cancer screening, LDCT without intravenous contrast is currently recommended (instead of standard-dose CT) to decrease the dose of radiation. Although there is no strict definition of LDCT of the chest, it is usually approximately 10% to 30% of standard-dose CT. In most cases, LDCT has been shown to be as accurate as standard-dose CT for detecting solid pulmonary nodules, although nodule detection with LDCT may be limited in larger patients” (NCCN, 2020). Further, “to help ensure good image quality, all LDCT screening programs should use CT scanners that meet quality standards equivalent to or exceeding the accreditation standards of the ACR” (NCCN, 2020). In the current NCCN guidelines, “using low-dose techniques, the mean effective radiation dose is 1.5 millisievert (mSv) (standard deviation [SD], 0.5 mSv) compared with an average of 7 mSv for conventional CT. . . . Lower doses of radiation are now used for LDCT scans and these lower doses may be less dangerous” (NCCN, 2020).
Low Dose CT Reporting and Management System
The current NCCN guidelines state that “to help ensure good image quality, all chest LDCT screening program should use CT scanners that meet the standards of the American College of Radiology (ACR). The ACR has developed Lung Imaging Reporting and Data System (Lung-RADS) to standardize the reporting and management of LDCT lung examinations” (NCCN, 2020). The NCCN guidelines go on to state that “the ACR developed Lung-RADS specifically for the lung cancer screening population in order to provide a standardized reporting and management tool for clinicians. Lung-RADS should be used, and not Fleischner Society Guidelines, when interpreting CT findings in an individual who has undergone lung cancer screening. . . . The NCCN Lung Cancer Screening Panel has harmonized Lung-RADS with the NCCN Guidelines for Lung Cancer Screening by revising the nodule management algorithm for screen-detected lung nodules” (NCCN, 2020).
Wood DE et al. National Comprehensive Cancer Network® (NCCN) Clinical Practice Guidelines in Oncology: Lung Cancer Screening. Version 1.2022 – October 26, 2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed December 21, 2021.
In the newly updated National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Lung Cancer Screening Version 1.2022, the NCCN Lung Cancer Screening Panel defined the risk status of “high risk” as “age ≥ 50 years and ≥ 20 pack-year history of smoking” with these high risk persons candidates for low dose CT (LDCT) screening (category 1) where “shared patient/provider decision-making is recommended, including a discussion of benefits/risks” (NCCN, 2020). For NCCN categories of evidence and consensus, Category 1 is defined as “based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate” (Wood, NCCN; 2021).
This section summarizes notable changes to the NCCN guidelines from 2020 to 2021.
- Evidence-based Guidelines Section: Within the algorithm image please note the most recent Guideline version has changed shared decision-making recommendation terminology from patient/physician to patient/provider.
- Changing Stopping Age From 77 years to 80 years Section: Removal of language stating that NCCN discusses uncertainty around Low Dose CT after 77 years.
- Smoking cessation section: Changing “smoking cessation counseling is recommended” to “current smokers should be advised to quit smoking, and former smokers should be advised to remain abstinent from smoking”.
- Please note that the NCCN Guidelines for Lung Cancer Screening contain detailed recommendations for evaluating and follow up on lung screening findings including immediate assessment of nodules that are highly suspicious for lung cancer. The NCCN Guidelines and the Lung-RADS recommendations have been
harmonized to provide consistent and clear recommendations to clinicians seeking to interpret LDCT scans.
(Wood, NCCN; 2021)
Professional Society Recommendations
AAFP (American Academy of Family Physicians). Clinical Preventive Service Recommendation: Lung Cancer: Lung Cancer Screening, Adult. https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/lung-cancer.html. Accessed 6/17/2021.
The American Academy of Family Physicians gives the clinical preventive services recommendation for lung cancer screening in adults a B grade recommendation. “The AAFP supports the United States Preventive Services Task Force (USPSTF) recommendation for annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery” (AAFP, 2021). The clinical preventive services recommendation further states that the “AAFP has reviewed the evidence and has determined there is sufficient evidence to support a recommendation for lung cancer screening in adults at increased risk. However, the AAFP acknowledges that the harms from annual screening with LDCT are not well documented at this time and that there are considerable barriers to screening for lung cancer in the community setting. Further research is needed to determine the harms of annual screening with LDCT including overdiagnosis, unnecessary procedures due to incidental findings, and barriers to care among communities of color (2021)” (AAFP, 2021).
Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013 Mar-Apr;63(2):107-17. doi: 10.3322/caac.21172. Epub 2013 Jan 11. PMID: 23315954
In 2013, before the 2021 updated USPSTF recommendation statement on lung cancer screening, the American Cancer Society issued an initial guideline for lung cancer screening. As of August, 18, 2021, the American Cancer Society website does not have a more recent updated lung cancer screening guideline other than the 2013 lung cancer screening guideline.
The American Cancer Society 2013 “guideline recommends that clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30 [or more]–pack-year smoking history and who currently smoke or have quit within the past 15 years. A process of informed and shared decision-making with a clinician related to the potential benefits, limitations, and harms associated with screening for lung cancer with low-dose computed tomography should occur before any decision is made to initiate lung cancer screening” (Wender, 2013).
The American Cancer Society has taken down the 2013 lung cancer screening guideline while it is being updated. The current guidance from the American Cancer Society is that health care providers and people at increased risk for lung cancer follow the recently updated recommendations for annual lung cancer screening from the US Preventive Services Task Force (USPSTF), the American Academy of Family Physicians (AAFP), or the American College of Chest Physicians. These organizations recommend yearly lung cancer screening with LDCT scans for people who: are 50 to 80 years old and in fairly good health, currently smoke or have quit in the past 15 years, and have at least a 20 pack-year smoking history. In addition, people who are going to be screened should receive counseling to quit smoking if they currently smoke, have been told by their doctor about the possible benefits, limits, and harms of screening with low dose CT scans, and can go to a center that has experience in lung cancer screening and treatment: (https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/detection.html).
Wiener RS, Gould MK, Arenberg DA, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015 Oct 1;192(7):881-91. doi: 10.1164/rccm.201508-1671ST. PMID: 26426785
Rivera MP, Katki HA, Tanner NT, et al. Addressing Disparities in Lung Cancer Screening Eligibility and Healthcare Access. An Official American Thoracic Society Statement. Am J Respir Crit Care Med. 2020 Oct 1;202(7):e95-e112. doi: 10.1164/rccm.202008-3053ST. PMID: 33000953
“The American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) convened a committee with expertise in lung cancer screening, pulmonary nodule evaluation, and implementation science. The committee reviewed the evidence from systematic reviews, clinical practice guidelines, surveys, and the experience of early-adopting LDCT screening programs and summarized potential strategies to implement LDCT screening programs successfully” (Wiener, 2015). Additionally, “this policy statement offers pragmatic strategies to assist medical centers and healthcare systems that seek to establish comprehensive low-radiation-dose computed tomography (LDCT) lung cancer screening programs that are safe and effective. The strategies listed herein address the nine core components of LDCT screening programs proposed by the American Thoracic Society (ATS) and American College of Chest Physicians (CHEST)” (Wiener, 2015).
The ATS/CHEST policy statement concludes that “during the implementation phase, programs should be attentive to establishing systems to screen the right patients at the right time, [and] to performing shared decision-making to help eligible patients decide whether to undergo screening” (Wiener, 2015).
The ATS/CHEST policy statement notes that core programmatic functions “often conducted by a midlevel provider serving as screening coordinator, but sometimes tasked to PCPs [primary care physicians] or other clinicians” can include “to counsel and communicate with the patient about screening, [such as] initial shared decision-making about whether to undergo LDCT screening” (Wiener, 2015).
4. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
A MEDCAC meeting was not convened on this issue.
VIII. Public Comment
Public comments sometimes cite published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information will not be made available to the public.
CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum. All comments that were submitted without personal health information may be viewed in their entirety by using the following link https://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=304.
Initial Public Comment Period: 05/18/2021 – 06/17/2021
During the 30-day comment period following the release of the tracking sheet, CMS received 170 comments. All of the commenters supported revising the NCD to align with the updated USPSTF recommendations for lung cancer screening with LDCT. Some commenters also requested additional updates such as eliminating or increasing the upper age limit and the quit smoking limit; revising or removing the counseling and shared decision-making requirement; and clarifying the radiology imaging facility criteria to ensure that independent diagnostic testing facilities (IDTF’s) qualify to perform lung cancer screening with LDCT.
Comments were provided by advocacy organizations, cancer centers, cancer coalitions, imaging facilities, researchers; with the majority of comments provided by physicians, radiologists and other healthcare professionals. Eight comments were provided by national associations/professional societies, including American Association for Thoracic Surgery (AATS), American Cancer Society (ACS), American Lung Association, a joint comment from The American Thoracic Society (ATS) and American College of Chest Physicians (CHEST), Association of Quality Imaging (AQI), Medical Imaging &Technology Alliance (MITA), National Comprehensive Cancer Network (NCCN), and Radiology Business Management Association (RBMA).
Second Public Comment Period: 11/17/2021-12/17/2021
During the 30-day comment period following the release of the proposed decision memorandum, CMS received 49 comments.
All of the commenters supported revising the NCD to expand beneficiary eligibility for lung cancer screening with LDCT. Commenters applauded CMS for reconsidering this NCD in a timely manner following the release of the updated USPSTF recommendation and being responsive to concerns from the stakeholder community. Many commenters disagreed with retaining the upper age screening limit and the 15-year quit smoking history in the beneficiary eligibility criteria. While the majority of commenters supported and appreciated simplifying the requirements for the counseling and SDM visit, many commenters supported removing SDM as a requirement altogether. A few commenters requested CMS strengthen rather than simplify the SDM criteria. Commenters supported expanding the type of provider eligible to furnish the counseling and SDM by removing the restriction that it must be furnished by a physician or non-physician practitioner but one commenter wanted to ensure that SDM conversations still included a patient’s healthcare provider. Commenters supported modifying the reading radiologist eligibility criteria; however, several commenters disagreed with the removal of the requirement for 300 chest CT acquisitions within three years. While the majority of commenters acknowledged that removing the imaging facility eligibility criteria would reduce barriers and expand access to care, some commenters were concerned that the standards and overall quality of lung cancer screening would be reduced. Specifically, some commenters did not agree with removing smoking cessation interventions from the imaging facility criteria or removing the requirement to submit data to a CMS-approved registry. Detailed summaries of all submitted comments with CMS responses are included below.
Comments (22) were provided by academic institutions, advocacy organizations, cancer centers, healthcare systems, consortia, medical technology manufacturers, a Medicare Contractor Medical Director, and researchers; 15 comments were provided by physicians and other healthcare professionals. Two comments did not identify an affiliation or profession and one comment identified themselves as a concerned citizen. Nine comments were provided by national associations/professional societies, including American Association for Cancer Research (AACR), American Cancer Society (ACS), American Lung Association, Association for the Treatment of Tobacco Use and Dependence (ATTUD), Association of Quality Imaging (AQI), Medical Imaging & Technology Alliance (MITA), National Comprehensive Cancer Network (NCCN), Society for Research on Nicotine and Tobacco (SRNT) and a joint comment from the requestor consisting of the GO2 Foundation for Lung Cancer, American College of Radiology® (ACR®) and Society of Thoracic Surgeons (STS).
Numerous references were submitted with public comments that were not available or considered for the proposed decision. These references have been reviewed and those that fall within the scope of this NCD as well as our literature search and review parameters have been considered in the analysis section.
Beneficiary Eligibility Criteria
Comment: All of the commenters supported revising the NCD to expand beneficiary eligibility for lung cancer screening with LDCT by reducing the starting screening age from 55 to 50 and lowering the pack-years tobacco smoking history from 30 to 20 years. This expansion will expand access to at-risk populations; specifically, females, African Americans, and underserved populations. This also enables expansion for screening in persons who have enrolled in Medicare early due to disability or End Stage Renal Disease (ESRD).
Response: We agree with the importance of prevention and screening. We appreciate the supportive comments for expanding lung cancer screening with LDCT for Medicare beneficiaries.
Upper Age Limit
Comment: Commenters disagreed with retaining the upper age screening limit of 77. Commenters either supported increasing the age from 77 to 80 to align with the USPSTF recommendation and to be consistent with private insurers or eliminating the upper age limit to mirror the NCCN guidelines. Commenters who supported removal of the upper age limit believe that providers should be allowed to determine if lung cancer screening is appropriate on an individual basis. Commenters claim that the upper age of 77 is not supported by clinical evidence and based on arbitrarily chosen age cutoffs in various lung cancer screening clinical trials.
Response: The USPSTF recommended the upper age limit of 80 years in their previous recommendations published in 2014 and in their most recent recommendation. The 2015 NCD included the upper age limit of 77 because there were no clinical trial data or evidence on adults over 77 years. For this reconsideration, we reviewed all the available evidence published from 2014 to the present to determine if there were new data to support increasing the upper age limit from 77 to 80. As noted in our evidence review, we found there is still no empirical data available to support lung cancer screening with low dose CT for adults aged 78-80 years. Data from randomized controlled trials provide the strongest evidence. While the main focus of the review was on randomized controlled trials, we did include relevant prospective cohort and cross-sectional studies that fell within the scope and inclusion parameters of our internal systematic review. There continues to be no relevant published human clinical study literature regarding the use of low dose CT in persons age 77- to 80-years-old and therefore, the evidence is insufficient to determine if patients over 77 years would benefit from low dose CT screening for lung cancer.
Comment: Commenters pointed out that CMS should consider modeling data and real-world data instead of or in addition to clinical trials for increasing the upper age from 77 to 80 or removing the upper age limit altogether. They state that while clinical trials are useful in developing levels of evidence, they do not reflect the real-world circumstances. They highlight that the USPSTF review was based heavily on modeling data.
Response: We agree that the available evidence in this upper age range of 78-80 years does not include empirical evidence and relies solely on modeling. We consider modeling as a very low level of evidence. In general, we believe data using statistical methods of modeling is inadequate to ensure that the service would be “reasonable and necessary for the prevention or early detection of an illness or disability” or be “appropriate” for Medicare beneficiaries. While simulation modeling can be valuable tools that contribute to our understanding of lung cancer screening with LDCT, there are challenges, such as uncertainty inherent about modeling and variability in the results depending on the assumptions used, and uncertainty around the usefulness and applicability of modeling studies. Thus, the modeling information provided by commenters was a very low level of evidence that we did not find persuasive to make changes in the eligibility criteria for lung cancer screening with low dose CT.
Evidence Review
Comment: A commenter supported our decision to not focus on meta-analysis studies in the evidence review. They believe there is too much heterogeneity in the existing RCTs of LDCT screening for meta-analysis results to be informative, and there is sufficient evidence in the small number of larger trials.
Response: We appreciate the supportive comment.
Comment: Two commenters responded to the need for additional studies in populations aged above 77. One commenter remarked that additional studies would likely take several years to conduct any new research and the other commenter suggested CMS conduct coverage with evidence development (CED).
Response: We agree with the need for additional evidence as our review concluded that there is an absence of high-quality evidence to support lung cancer screening above age 77 with low dose CT. As stated in our evidence review, data from randomized controlled trials provide the strongest evidence. In response to the commenter suggesting CMS initiate coverage with evidence development (CED), our review, as stated above, determined that there was a lack of evidence as opposed to identifying that the evidence is promising in that age group. It should be noted that the paradigm around CED is that the evidence is promising in demonstrating that an item or service is reasonable and necessary. Therefore, in the absence of relevant published human clinical study evidence, populations aged above 77 will remain non-covered by Medicare.
15-Year Quit Smoking History
Comment: Commenters disagreed with retaining the beneficiary eligibility requirement of having quit smoking within the past 15 years. They support removal of the criteria or at a minimum, to adopt the NCCN guidelines. Many claimed there is no substantive data to support a significant reduction in lung cancer risk in this time frame while others state that multiple studies have concluded that there are benefits beyond the 15-year cut-off. Some claim that the 15-year cut-off is not based on or justified by evidence, rather that the USPSTF and CMS adopted it because it was an eligibility criterion in the National Lung Screening Trial (NLST). Another commenter expressed that we are misrepresenting RCT enrollment criteria as the reason to support the 15-year cut-off. They further state that it is well-known that these RCTs were not intended or designed to limit screening eligibility. Another commenter believed this criterion unintentionally incentivizes Medicare beneficiaries to continue or resume smoking, while another commenter stated it is difficult to obtain a quit date from patients.
Response: The 2021 USPSTF recommendation determined with a grade B rating that screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. The studies used to inform the USPSTF recommendation did not examine patients who have a quit smoking history greater than 15 years. We did not identify evidence to determine if persons who quit smoking more than 15 years ago would benefit from low dose CT screening for lung cancer. Therefore, in the absence of relevant evidence, beneficiaries who have quit smoking greater than 15 years ago remain non-covered by Medicare.
Several studies have been cited as purportedly showing benefit of lung cancer screening with low dose CT for the 15-year Quit Smoking History criterion. For instance, the objective of the Tindle et al. (2018) investigation was “to relate comprehensive lifetime smoking history to the risk of lung cancer in a large and well-characterized prospective cohort study, the Framingham Heart Study (FHS), which began in 1948 with enrollment of the Original cohort.” However, this study did not assess the relevant and important health outcome of mortality, instead assessing the risk of developing lung cancer. Additionally, the Framingham Heart Study did not investigate screening with low dose CT.
Two other studies (Reitsma, 2020; Wang, 2015) did not assess mortality and did not investigate screening with low dose CT. The objective of the Reitsma et al. (2020) study was to “characterize the percentage of the reducible relative risk (RR) remaining for lung cancer as a function of years since quitting (YSQ)”. The objective of the Wang et al. (2015) study was to investigate the “implications of the USPSTF screening criteria . . . in a well-defined population retrospectively over 28 years to demonstrate trends in the proportion of patients with lung cancer meeting the criteria”.
Additionally, the objective of the McKee et al. (2018) study was to “assess the performance of patients in NCCN high-risk group 2 in a clinical CT lung screening (CTLS) program”. However, this study did not assess the health outcome of mortality, instead assessing the risk of developing lung cancer. The overall objective of the Luo et al. (2019) was to directly compare “overall survival between patients with lung cancer meeting USPSTF screening criteria and those who are ineligible because they are classified as long-term quitters or in a younger age group at the time of lung cancer diagnosis”. This approach allowed Luo et al. (2019) to assess survival outcomes in two subgroups: either long-term quitters (≥15 years since quitting) or from a younger age group (age 50–54 years). However, the Luo et al. (2019) study did not investigate lung cancer screening with low dose CT in this prospective cohort study.
While the commenters disagreed with retaining the beneficiary eligibility requirement of having quit smoking within the past 15 years, we concluded that the studies discussed in the prior paragraphs were not sufficient to remove the 15-year quit smoking criterion because the studies did not investigate screening with low dose CT or did not assess the important health outcome of mortality. Thus, we are not adopting the commenters suggestions and will retain the beneficiary eligibility requirement of having quit smoking within the past 15 years.
Written Order
Comment: Commenters supported the removal of the word ‘written’ from the written order requirement and the removal of the documentation requirement in the beneficiary’s medical record. They stated that written orders are time consuming and electronically transmitted orders are more efficient and improve overall patient compliance. An electronic order is more likely to generate patient follow-up for scheduling and care coordination.
Response: We appreciate the supportive comments. We believe that eliminating the excessive requirement for a written order will reduce administrative burden and facilitate improved access to lung cancer screening with LDCT.
Other
Comment: Several commenters suggested expanding the beneficiary eligibility criteria beyond the USPSTF recommendation to include additional risk populations. Commenters suggested CMS should consider how lung cancer screening can further reach at-risk Medicare beneficiaries who have no smoking history but have other potential risk factors including long-term exposure to secondhand smoke, and environmental and occupational exposures. Commenters also noted that the current beneficiary eligibility criteria do not consider beneficiaries who smoked less than one pack per day or smoked for less than 20 years, including beneficiaries with occupational exposures, such as first responders.
Response: In order for an additional preventive service to be included for Medicare coverage, it must be recommended with a grade of A or B rating by the USPSTF. The current USPSTF Recommendation (2021) assigned a grade B to adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. A grade B rating determines there is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. The studies used to inform the USPSTF recommendation either did not examine these additional at-risk populations or there was no evidence or the evidence did not support an A or B rating. The USPSTF considers all populations that are supported by the available evidence within its analytic framework of the systematic review for lung cancer screening with low dose CT. Thus, we are not adopting the commenters suggestion to expand the beneficiary eligibility criteria to include additional risk populations.
Comment: One commenter suggested we incorporate using an accurate risk prediction model in the screening criteria.
Response: The risk prediction modeling information provided by commenters was a very low level of evidence that we did not consider sufficient to make changes in the eligibility criteria for lung cancer screening with low dose CT.
Counseling and Shared Decision-Making Visit
Comment: While the majority of commenters supported and appreciated CMS simplifying the requirements for the counseling and SDM visit, many commenters supported removing counseling and SDM as a requirement altogether. They stated that SDM should be encouraged and utilized but eliminated as a documentation requirement for coverage and reimbursement. They believe that even the simplified requirements will pose administrative burden and may create a barrier to screening. Some commenters noted that other screening services such as mammography and colon cancer screening do not have the same SDM requirement and that the USPSTF only recommends an SDM visit prior to lung cancer screening.
Other commenters requested CMS strengthen rather than simplify the SDM criteria. They believe currently there is too much flexibility in how these discussions are framed and state that prior evidence has shown SDM to be sub-optimal. The commenters request that CMS retain the documentation of beneficiary eligibility requirements and should also provide explicit guidance related to key benefits and risks for the counseling and SDM visit.
Several other commenters supported simplifying the complexity and number of requirements within the counseling and SDM visit. They believe this is a step in the right direction to reducing barriers to screening to create broader uptake of lung cancer screening. One commenter stated that cancer care is a team effort between the patient, their families and the entire clinical staff and these discussions are critical.
Response: We appreciate the commenters recognizing the importance of counseling and SDM in lung cancer screening with LDCT and understand the additional concerns expressed. We agree that SDM is a critical component of lung cancer screening with LDCT due to the complexities around the discussion of low dose CT and benefits, harms and patient adherence, therefore SDM will remain a requirement within the NCD. However, we believe that this service has matured since the original 2015 NCD and the documentation requirements can be simplified. Professional societies and provider groups have noted that providers have gained considerable experience over the years. We believe by removing some specificity around documentation of the information on the beneficiary eligibility criteria and SDM elements, it will reduce provider burden and thus, likely improve access to lung cancer screening with low dose CT.
The USPSTF includes their support for SDM in the ‘Practice Guidelines’ section of the Recommendations. Due to the fact that lung cancer screening has the potential to cause harm, including false-positive screening test results and the risks of overdiagnosis of lung cancer and excess radiation exposure, they state the decision to undertake screening should involve a thorough discussion of the potential benefits, limitations and harms of screening. We agree that SDM is a critical component of lung cancer screening with LDCT and therefore, will remain as a requirement.
Comment: Several commenters who favored removal of the counseling and SDM visit requested that if CMS chose to retain the requirement, telehealth should be allowed. They pointed out that as part of the COVID-19 Public Health Emergency (PHE) Blanket Waivers for Healthcare, shared decision-making was considered a telehealth service. These commenters advocated for telehealth to be permanently adopted for the shared decision-making visit.
Response: We appreciate the comments and support reducing barriers and increasing access through telehealth. CPT code G0296, defined as a Counseling visit to discuss need for lung cancer screening (LDCT) using low dose CT scanning (the service is for eligibility determination and shared decision making), is listed as a permanent telehealth code. The code is payable in the facility and the non-facility setting. You can access the complete list of telehealth services at: https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
Comment: Two commenters who favored removal of the counseling and shared decision-making visit requested that if CMS chose to retain the requirement, CMS should consider allowing the shared decision-making visit to occur in conjunction with the actual lung cancer screening exam rather
than requiring a separate visit.
Response: The NCD states that the counseling and shared decision-making visit must occur before the beneficiary’s first lung cancer screening. The NCD does not prevent the SDM visit from occurring on the same day as the lung cancer screening exam or from occurring in conjunction with the actual lung cancer screening exam. As long as the counseling and shared decision-making visit occurs before the beneficiary’s first lung cancer screening exam then it satisfies the NCD. As a reminder, this required counseling and shared-decision making visit is only required prior to the first LDCT screening.
Comment: One commenter believes that patients’ current low level of screening may be caused by former smokers, who know they are at higher risk, being fearful of diagnosis, especially if they have loved ones who died of lung cancer. For this reason, they urge CMS to ensure that patients receive accurate, balanced, unbiased information on the benefits and risks of lung cancer screening.
Response: We appreciate the comment. In addition to the importance of SDM addressing the benefits and harms of lung cancer screening, the counseling and SDM visit includes counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment; and counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions. One underlying purpose of the counseling and shared decision-making visit is for the patient to have a complete and fully comprehensive discussion with the health care provider that thoroughly addresses all of the concerns of the patient.
Furnished by Physician or Non-physician Practitioner (NPP)
Comment: Commenters supported the removal of the requirement that the counseling and shared decision-making visit must be furnished by a physician or non-physician practitioner (NPP) while one commenter wanted to ensure that shared decision-making conversations still include a patient’s healthcare provider. This expansion allows other types of providers eligibility to furnish this service. Commenters acknowledged this expansion will ease the burden on physicians and NPPs and expand the number of providers who can deliver the counseling and shared decision-making visit. One commenter urged CMS to maintain that the shared decision-making visit should include a patient’s healthcare provider. While they acknowledge that health educators and others who are specifically trained to discuss the risks and benefits of LDCT and can spend more time with patients than physicians, they should not do so without the inclusion of healthcare professionals. They suggest that improving training for the shared decision-making conversations with the physicians and NPPs could be a possible solution rather than eliminating the requirement altogether.
Response: We appreciate the support and understand the concern to include the beneficiary’s healthcare provider. While we support the need for shared decision-making, we acknowledge that lung cancer screening with LDCT is no longer considered a new service. Professional societies and provider groups have noted that providers have gained considerable experience and expertise. We believe flexibility will reduce burden and therefore are removing the requirement that the counseling and shared decision-making visit must be furnished by a physician or NPP. This expansion can allow for this service to be furnished by auxiliary personnel “incident to” a physician’s professional service. Therefore, there still exists a relationship between the physician or NPP and the auxiliary personnel furnishing the SDM visit as there are existing rules that apply. We believe this flexibility will enable a more streamlined process and broaden access to LDCT screening. We also support continued training for shared decision-making for all healthcare providers and encourage providers to consult with professional societies and provider groups for training opportunities.
Decision Aids
Comment: Commenters generally supported the requirement that shared decision-making include one or more decision aids but cited the need for more specificity; while one commenter favored the requirement be removed. One commenter requested that the requirement specifically state that decision aids should provide patient education on the pros and cons of screening and another commenter suggested the decision aid include a checklist to be initiated by the patient to acknowledge the conversation took place. One commenter stated that inaccurate decision aids should be discarded and replaced with materials that provide timely and accurate information on benefits and risks of lung cancer screening. Another commenter reported that decision aids increase efficiency and ensure the key information is communicated. They informed us that the ACS-National Lung Cancer Roundtable is collaborating with the American Academy of Family Physicians to develop second-generation decision aids. The commenter who favored the removal of the requirement to use decision aids stated that since there is no designated, standard decision aid it creates more complexity and administrative burden for providers.
Response: To reduce the burden of documentation paperwork for providers, we are removing the specifications for the components of the shared decision-making tools. As noted in the Analysis, several professional organizations and societies suggest the use of decision aids but do not specify the elements required as part of the shared decision-making tool. While we believe the tools and guidance have matured since the early inception of shared decision-making, we are continuing not to require the use of a specific decision aid. Eligible practitioners may select from various available decision aids designed for this purpose and recognized by national professional medical organizations. We appreciate and are encouraged by the information regarding the progress and development of decision aids.
Counseling on the Importance of Adherence to Annual Lung Cancer LDCT Screening
Comment: A commenter requested we improve this requirement by making a slight modification to the wording. The request was to change, “Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment” to “Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability and willingness to undergo diagnosis and treatment.” The commenter pointed out that it is both illogical and wasteful to perform a screening test if it is known beforehand that the beneficiary is unwilling.
Response: We appreciate the commenter pointing out this wording. We note that the language of the NCD is consistent with the “or” used in the USPSTF Recommendation. We are not aware that this language is a cause of confusion for ordering practitioners or providers, therefore, to remain consistent with the wording of the USPSTF recommendation, we will retain the sentence as written.
Reading Radiologist Eligibility Criteria
Documented Training
Comment: Commenters supported the removal of the training documentation requirement. They believe training is basic and also included in Maintenance of Certification (MOC) for reading radiologists.
Response: We appreciate the supportive comments. We believe removing this redundant requirement will reduce documentation burden on the provider.
300 Chest CTs within the Past 3 Years
Comment: Several commenters did not support the removal of the requirement for reading radiologists to document involvement in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years. They believe it is not a prohibitive requirement and it shows that the reading radiologist has some prior experience in screening images, ideally accompanied by mentoring. Two commenters mentioned that there are no provisions to require the American College of Radiology to mandate any certification or training for radiologists to read LDCT scans which would ensure a high-quality LDCT report.
Response: LDCT screening, as we have stated elsewhere in this document, is a more mature service than when CMS first issued an NCD and it is no longer necessary for CMS to dictate experience as part of the NCD. We believe high quality reporting will continue as training, including formal training, is more readily available to radiologists. Imaging centers or professional societies may choose to adopt an experiential requirement but eliminating this issue from the NCD also reduces documentation requirements as part of coverage.
CME Requirement
Comment: A commenter requested clarification as to why the documentation requirement for participation in continuing medical education (CME) in accordance with current ACR standards was retained within the Reading Radiologist Eligibility Criteria. They state that the NCD does not specify any requirement for CME specific to lung cancer screening within the 75 CME credits required for Maintenance of Certification (MOC) and it is the same required documentation needed to submit to the ACR and ABR.
Response: We appreciate the commenter identifying this discordance. We have more carefully reviewed the language in this requirement and have concluded that the requirement is not meaningfully contributing to the policy because it is unclear and nonspecific. Further, board certification currently requires continuing education leaving it unnecessary to maintain this separate requirement. We are removing this requirement in our final decision.
Radiology Imaging Facility Criteria
Radiation Dose
Comment: Several commenters did not support the removal of the radiation dose criteria. They believe that eliminating this requirement could potentially cause confusion and no longer provide a distinction between low dose and regular dose screening protocols.
Response: An important aspect of LDCT screening is reducing radiation exposure to as low as reasonably achievable especially given the annual screening frequency and likely follow-up diagnostic imaging tests. When the 2015 NCD was finalized, the acquisition parameters for low-dose CT were not explicitly defined in the imaging community, so the radiation dose requirement as part of the NCD was essential. Multi-society, multi-disciplinary organizations with extensive expertise in LDCT scans, such as the American College of Radiology, have published guidelines on the radiation dose that should be emitted by the low dose CT scan. The guidelines also appear to support the effort to standardize the protocol for administering the low dose CT scan. Given that there are readily available evidence-based guidelines and LDCT screening is now a mature technology, we are removing the radiation dose requirement from the NCD. This also allows guidelines to adapt, as needed. Requirements in an NCD remain static and could become outdated. Thus, we are not adopting the commenters’ suggestion and will remove this requirement from the NCD as proposed.
Lung Nodule Reporting System
Comment: Some commenters did not support the removal of the requirement for utilization of a standardized lung nodule identification, classification and reporting system. They noted that Lung-RADS is an example of a system that is deemed appropriate by the lung cancer screening community and generally the one most used. The commenters believe that removing the standardized reporting system could impact the quality of lung cancer screens and affect patient management and care by increasing ambiguity in follow up and clinical management recommendations made to ordering providers. They stated that Lung-RADS provides clear and consistent communication between the radiologist and the ordering providers by helping ordering providers decide on appropriate follow-up for suspicious results. Another commenter was concerned that this will provide opportunity for home-grown reporting systems and entrepreneurial efforts by competing groups, and undermine the efforts of everyone using a common system.
Response: After considering the public comments, we did not finalize our proposal to remove the requirement for utilization of a standardized lung nodule identification, classification and reporting system as part of the radiology imaging facility eligibility criteria. We believe that utilizing a standardized lung nodule identification, classification and reporting system is likely to standardize low dose CT screening and the evaluation and management of abnormal lung nodule findings and to provide clear and consistent communication between the reading radiologist and the ordering providers. Additionally, the USPSTF notes in their low dose CT lung cancer screening recommendation that data suggest that the use of Lung-RADS may decrease the rate of false-positive results in lung cancer screening (USPSTF, Krist; 2021). Therefore, after further review, utilization of a standardized lung nodule identification, classification and reporting system, such as Lung-RADS, will remain a requirement for a radiology imaging facility.
Smoking Cessation Interventions
Comment: Some commenters did not support removing the requirement for smoking cessation interventions for current smokers from the radiology imaging facility criteria. They state that it is appropriate and essential to receive smoking cessation interventions at every touchpoint in the lung cancer screening process. They believe that CMS is being contradictory by stating that smoking cessation interventions are vitally important at every touchpoint between the provider and patient along the clinical pathway for lung cancer screening, yet we are removing the requirement from the radiology imaging facility criteria. Other commenters did not agree with CMS stating that it is not appropriate for Medicare beneficiaries who are current smokers to receive smoking cessation interventions within the radiology imaging facility setting and therefore removing it as a requirement. A few commenters provided specific examples of smoking cessation interventions that CMS could require of the radiology imaging facilities.
Other commenters supported the removal of this requirement and applauded CMS for streamlining the process to allow independent diagnostic testing facilities (IDTFs) the ability to perform and receive reimbursement for lung cancer screening. Since IDTFs are enrolled as a facility for the purpose of performing diagnostic tests and smoking cessation interventions are considered therapeutic interventions, they did not satisfy the NCD criteria and were unable to receive reimbursement for the lung cancer screening service. Commenters believe the removal of this requirement expands access to lung cancer screening by increasing accessibility to screening sites.
Response: CMS strongly supports all opportunities to provide appropriate smoking cessation services to beneficiaries. It was not our intent to convey a message that implied otherwise. Removing the requirement for imaging facilities to furnish smoking cessation services was not intended to downplay the importance of this intervention but rather to expand the availability of LDCT screening. There are separate rules and regulations that determine the services that are legally able to be furnished in certain Medicare recognized settings. We determined that while smoking cessation services are of critical importance we have a need to balance the accessibility of LDCT screening. While smoking cessation services are appropriate for patients, we are not making it a requirement that imaging facilities furnish the service because it would prevent IDTFs from furnishing LDCT screening. We have updated the language in the analysis section in order to correct any misunderstanding that may have implied that CMS was not supportive of smoking cessation services.
In order to avoid any further misunderstanding, we have revised and clarified the language used in the analysis. Specifically, we have removed the word “appropriate” from the final decision memorandum Section IX (CMS Analysis, Smoking Cessation) and replaced it with “required”, indicating (as we have noted), that as a policy requirement for low dose CT screening NCD, a smoking cessation intervention is not a radiology imaging facility eligibility criterion. The sentence now reads as, “it is not required for Medicare beneficiaries to receive smoking cessation interventions for current smokers within the setting of a radiology imaging facility.” It remains critically important to provide information about tobacco cessation or cigarette smoking abstinence to the patient at touchpoints between provider and patient along the clinical pathway.
CMS-Approved Registry
Comment: Some commenters did not support the removal of the requirement for radiology imaging facilities to submit data to a CMS-approved registry for each LDCT lung cancer screening
performed. Commenters noted that the registry provides guidance for required and optional fields, facilitates additional research, aids in quality audits, quality improvements and establishes best practices. They are concerned that removing this requirement will likely result in lower overall quality of lung cancer screening. One commenter agreed with the original purpose of the registry as outlined in the proposed DM, but believes the three published studies reinforced the purpose of the registry rather than fulfilled it.
Other commenters supported the removal of the registry requirement stating that it creates a financial disincentive to small radiology imaging facilities and a barrier to overall lung cancer screening, particularly for rural and minority populations. They point out that the only CMS-approved lung cancer screening registry requires that providers pay a fee to input their data into the registry. The commenters believe by removing this requirement it will expand access by encouraging more providers to offer lung cancer screenings.
Response: We appreciate the comments. The primary purpose for requiring the submission of data to a CMS-approved registry, as stated in the 2015 Decision Memo, was “to document compliance with the coverage criteria that are not evidenced on the health care claim. The registry will help ensure that only eligible beneficiaries will receive this screening service since only beneficiaries that meet the eligibility requirements will benefit from such screening.”
In addition, the most recent 2021 USPSTF recommendation statement changed from encouraging the development of a registry in 2014 to having no comment on the need for a lung cancer registry. Since the published studies, using data from the LCSR, fulfilled the purpose as outlined in the previous NCD, it is no longer necessary to mandate data collection through this NCD. This will also reduce the administrative burden on providers and institutions.
Comment: Several commenters supported the development of a free lung cancer screening registry.
Response: We agree that a registry that would be free to access would aid in eliminating the financial burden for smaller providers. We are aware of a proposed bill in Congress (H.R. 107-The Lung Cancer Screening Registry and Quality Improvement Act of 2021) that if passed would provide grant funding for the development of free lung cancer screening registries and encourage the development of quality measures in lung cancer.
Comment: Two commenters suggested CMS make the Lung Cancer Screening Registry (LCSR) data publicly available.
Response: The LCSR is maintained by the American College of Radiology (ACR). We encourage our commenters and any interested party to reach out directly to the ACR for more information about data access.
Additional Comments
Comment: The American Cancer Society (ACS) and the National Comprehensive Cancer Network (NCCN) requested we update their respective sections within the Decision Memorandum (DM). ACS informed us that their 2013 guideline is currently being updated and has been taken down from their website. They provided interim language and asked that we replace our language in the final DM. NCCN provided us with the most recent version of the NCCN Guidelines and requested we update the risk assessment image in the final DM.
Response: We thank ACS and NCCN for advising us of the current information. We updated the ACS and NCCN sections within the Evidence-based Guidelines section of the DM to reflect their requests.
Comment: Several commenters advocated that lung cancer screening be added to hospital performance and quality measures in such reporting systems such as the Healthcare Effectiveness Data and Information Set (HEDIS), Merit-based Incentive Payment System (MIPS), and National Quality Forum (NFQ).
Response: We thank the commenters, but quality measures are beyond the scope of this NCD.
Comment: One commenter raised concerns regarding the April 30, 2014 Medicare Evidence Development & Coverage Advisory Committee (MEDCAC) meeting.
Response: The 2014 MEDCAC meeting is not part of this reconsideration. Details regarding this meeting are summarized in the 2015 DM. A MEDCAC meeting was not convened for this reconsideration.
Comment: One commenter requested to include ICD-10 diagnosis codes for ‘smoking in remission.’
Response: Coding guidance is outside the scope of this national coverage analysis.
Comment: One commenter asked when the changes are effective.
Response: All NCDs are effective on the date the final decision memoranda are posted.
Comment: One commenter urged CMS to work with appropriate federal and state stakeholders to address additional barriers that exist for both providers and patients, including ensuring private insurance plans include lung cancer screening as a routine screening for eligible patients; including eliminating LCS as a cost-sharing benefit and to urge the American Academy of Family Physicians (AAFP) to acknowledge the importance of lung cancer screening in eligible patients, thus raising their grade on lung cancer screening to “A” from its current ranking of “B”.
Response: While CMS does not have direct authority to influence these changes, we agree that continued stakeholder engagement is important. The updated NCD may contribute to opportunities for continued stakeholder engagement to address additional barriers to access to lung cancer screening.
IX. CMS Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act (§ 1869(f)(1)(B)). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. CMS is authorized to cover "additional preventive services" if certain statutory requirements are met as provided under § 1861(ddd) of the Social Security Act. Regulations at 42 C.F.R. § 410.64 provide:
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Services Task Force.
(3) Appropriate for individuals entitled to benefits under part A or enrolled under
Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
When making national coverage determinations, it is important to consider whether the evidence is relevant to the Medicare beneficiary population. In considering the generalizability of the results of the body of evidence to the Medicare population, it is necessary to consider at least the age, race and gender of the study participants.
Due to the natural history of lung cancer, the burden of lung cancer is high in older adults. In this analysis, we will evaluate specific criteria of screening tests as described by Cochrane and Holland (1971) in addition to the hierarchical framework of Fryback and Thornbury (1991). We will utilize the hierarchical framework of Fryback and Thornbury (1991) in our analysis of low dose CT screening tests for lung cancer. As Cochrane and Holland (1971) noted, “evidence on health outcomes, for example, evidence that screening can alter the natural history of disease in a significant proportion of those screened," is important in the consideration of screening tests since individuals are asymptomatic and "the practitioner initiates screening procedures." We will look for sound human clinical evidence that shows that changes in the eligibility criteria for a screening test has clinical utility since lung cancer screening with low dose CT has been shown to improve health outcomes, including lung cancer mortality, which benefits Medicare beneficiaries.
Evidence Review Summary:
For this reconsideration of the NCD on lung cancer screening with low dose CT, CMS focused on three questions:
Question 1: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is recommended with a grade of A or B by the United States Preventive Services Task Force?
In 2021, “[t]he USPSTF recommends annual screening for lung cancer with LDCT in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (USPSTF, Krist; 2021). “This recommendation replaces the 2013 USPSTF statement that recommended annual screening for lung cancer with LDCT in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years” (USPSTF, Krist; 2021).
Question 2: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is reasonable and necessary for the prevention or early detection of illness or disability?
The human empiric evidence was assessed to determine whether changes in the beneficiary eligibility criteria for age and smoking history when screening with low dose CT show benefit for Medicare beneficiaries in the prevention or early detection of lung cancer by lowering lung cancer mortality.
Randomized Controlled Clinical Trials
The evidence base for this reconsideration of low dose CT for lung cancer screening has a primary focus on randomized controlled clinical trials of the effect of screening with low dose CT on the outcome of lung cancer mortality. Ten randomized controlled clinical trials involving human subjects were included in the evidence: the Continuous Observation of Smoking Subjects trial (COSMOS; Rampinelli, 2017), the Danish Lung Cancer Screening Trial (DLCST; Wille, 2016), the Detection And screening of early lung cancer with Novel imaging Technology and molecular assays trial (DANTE; Infante, 2015), the Italian Lung Cancer Screening Trial (ITALUNG; Paci, 2017), the Lung Cancer Screening Intervention trial (LUSI; Becker, 2020), the Lung Screening Study (LSS; Doroudi, 2018), the Multicentric Italian Lung Detection study (MILD; Pastorino, Silva, Sestini, 2019), the National Lung Screening Trial (NLST; National Lung Screening Trial Research Team [NLST], 2019), the Nederlands–Leuvens Longkanker Screenings Onderzoek trial (NELSON, which is the Dutch acronym; de Koning, 2020), and the United Kingdom Lung Cancer Screening pilot study trial (UKLS; Field; May, 2016). In subsequent discussions, each randomized controlled trial will be referred to by its acronym.
Of the 10 randomized controlled trials that report on lung cancer mortality (Table 1), only the NLST and the NELSON trials were adequately powered to answer the question of whether a mortality benefit from screening with low dose CT can be achieved (USPSTF, Krist; 2021). None of the other trials were individually powered to adequately address a mortality benefit due to issues such as a smaller sample size or the screening of a lower risk group than the NLST. Among the 10 randomized trials, the NLST (n = 53,454) and NELSON (n = 15,792) studies were the largest randomized controlled trials. Compared to the evidence cited in the 2015 national coverage determination on low dose CT, four studies provided longer follow-up results for the Dutch Belgian NELSON, the Italian MILD, the German LUSI, and the United States NLST trials. For benefits of screening, the NLST demonstrated a reduction in lung cancer mortality and all-cause mortality with three rounds of annual low dose CT screening compared with chest radiography, and the NELSON trial demonstrated a reduction in lung cancer mortality with four rounds of LDCT screening with increasing intervals (Jonas, 2021). “Results of the other trials [besides NLST and NELSON, such as LUSI and DLCST] were very imprecise and did not show statistically significant differences between groups” for lung cancer mortality (Jonas, 2021). “NLST and NELSON participants were younger, more highly educated, and less likely to be current smokers than the US screening-eligible population” (Jonas, 2021). Thus, the “general US population eligible for lung cancer screening may be less likely to benefit from early detection compared with NLST and NELSON participants because they face a high risk of death from competing causes, such as heart disease and stroke” (Jonas, 2021).
Modeling Studies
While simulation modeling can be valuable tools that contribute to our understanding of lung cancer screening with low dose CT, there are challenges, such as uncertainty inherent about modeling and variability in the results depending on the assumptions used, around the usefulness and applicability of modeling studies. Thus, modeling studies were not included in our internal technology systematic review of the published primary evidence involving human subjects for changes in the eligibility criteria for lung cancer screening with low dose CT.
Meta-Analyses
Four meta-analyses by Huang et al. (2019), Sadate et al. (2020), Wang et al. (2018), and Yang et al. (2019) were not the focus of the evidence base for this reconsideration of low dose CT for lung cancer screening due to concerns over substantial clinical and methodological heterogeneity (Table 2). The trials of lung cancer screening differed in eligibility criteria, such as the inclusion criteria for age, pack-years of smoking history, and years since quitting smoking, as shown in Table 2 and Table 3. As such, the heterogeneity of the study designs and results should be considered when interpreting the collective study results. As an example, the meta-analysis by Wang et al. (2018) was excluded because the authors reported a high degree of heterogeneity across the four studies included in the meta-analysis, “[t]he high level of heterogeneity between studies is a limitation to the ability to extrapolate results from this meta-analysis to the general population” (Wang, 2018). The authors go on to state other limitations in that the “trials varied in their enrollment criteria especially in the age of participants and level of tobacco exposure. They also varied in size; the largest study randomized more than 50,000 participants while the other three combined randomized fewer than 10,000. Also, the trials differed in their follow-up periods even among study arms as in the MILD trial. In addition, the smaller trials demonstrated heterogeneous study methods, selection criteria, and screening modalities. The study outcomes may also vary due to location, participant demographics, and differences in healthcare systems between the international locations of the trials.…” (Wang, 2018). The Huang (2019) study authors reported as a limitation that “there are major geographic differences, particularly in Asia, where 60 to 80% of women with lung cancer are never-smokers. In Yang 2018 study , they enrolled fewer active smokers (21.5%) and males (46.8%) than other USA and European trials. Although smoking is the primary etiologic factor responsible for lung cancer, racial/ethnic and sex differences may exist. According data from WHO, age-standardized rate of current tobacco smoking among population aged ≥15 years were estimated 2.2% for female in South-East Asia. Whereas for female in Americas and Europe, the rate were [sic] 12.4 and 20.7%. Previous studies also indicated that lung cancer [sic] significantly associated with Asian non-smoking women” (Huang, 2019).
There was considerable variability in the definition of the inclusion criterion for age across the four meta-analyses. For the Huang (2019) meta-analysis, the lower age limit of the inclusion criterion for age of the nine trials ranged from 45 to 60 years; and for the Sadate (2020), Wang (2018), and Yang (2019) meta-analyses, the lower limit for age ranged from > 49 to 60 years. For the Huang (2019) meta-analysis, the upper age limit of the inclusion criterion for age of the nine trials ranged from 69 to 75 years; for the Sadate (2020) meta-analysis, the upper limit for age ranged from 69 to 74 years; and for the Wang (2018) and Yang (2019) meta- analyses, the upper age limit ranged from 70 to 74 years.
The results of the four meta-analyses demonstrated heterogeneity in the effect of low dose CT on lung cancer mortality. For the Wang (2018) study, pooling data from the four studies showed no significant difference (odds ratio = 1.13, 95% CI: 0.78-1.64; I2 > 87.2%, p < 0.01, with I2 of 50-75% representing high heterogeneity among the studies; Wang, 2018) in lung cancer mortality between the LDCT screening group compared to other screening tools. The Yang (2019) meta-analysis results demonstrated that LDCT screening with up to 9.80 years of follow-up was associated with a statistically non-significant decrease in lung cancer mortality (pooled relative risk [RR] 0.94, 95% confidence interval [CI] 0.74 to 1.19; p = 0.62; Yang, 2019). A moderate level of heterogeneity was observed in the magnitude of effects (I2 = 43.3%, p = 0.152; Yang, 2019). However, in the pooled analysis for the Huang (2019) study, low dose CT reduced lung cancer mortality (risk ratio 0.83, 95% CI 0.76–0.90, I2 = 1%) with no heterogeneity observed in the meta-analysis (p = 0.43, I2 = 1%; Huang, 2019). For the Sadate (2020) meta-analysis, a significant relative reduction of lung cancer-specific mortality of 17% (risk ratio [RR] = 0.83, 95% confidence interval [CI]: 0.76-0.91) was observed in the screening group compared with the control group. There was no heterogeneity in the data (I2 = 0%, p = 0.67; Sadate, 2020). Median follow up ranged from three years to ten years. Thus, while the four meta-analyses, Huang (2019), Sadate (2020), Wang (2018), and Yang (2019), could contribute to improving our understanding of the effect of screening with low dose CT on lung cancer mortality, given the substantial clinical and methodological heterogeneity inherent in the meta- analyses, the four meta-analyses were not the focus of the evidence base for this reconsideration of low dose CT for lung cancer screening.
Lowering Starting Age From 55 years to 50 years
The 2021 USPSTF recommendation on lung cancer screening recommends that people start screening at age 50 (USPSTF, Krist; 2021), rather than age 55 years. The previous 2013 USPSTF recommendation started screening with low dose CT at age 55 years (Moyer, USPSTF; 2014). To determine whether to lower the starting age for low dose CT screening from 55 years to 50 years, we will assess the human clinical evidence on low dose CT screening and its impact on lung cancer mortality among persons age 50 to 54 years.
The study population for the Nederlands–Leuvens Longkanker Screenings Onderzoek (NELSON) study, one of two high quality randomized controlled clinical trials of low dose CT with a large sample size, the other being the National Lung Screening Trial (NLST) study, enrolled individuals between 50- and 55- years-old (de Koning, 2020). The NELSON study was a Dutch Belgian lung cancer screening study that consisted of 13,195 men, which was the primary analysis, and 2,594 women, which was the subgroup analysis, between the ages of 50 and 74 years who were at high risk and were randomly assigned to undergo low-dose computed tomographic (LDCT) screening compared to no screening (de Koning, 2020). The subgroup of men aged 50 to 54 years consisted of 24.6% (1611/6560) of the screening group. Among men age 50 to 54 years at randomization, there was a non-statistically significant 15% reduction in lung cancer mortality, since the rate ratio for death from lung cancer at 10 years of follow up was 0.85 (95% confidence interval [CI], 0.48 to 1.50) in the screening group as compared with the control group (de Koning, 2020). There was a strong trend, though not a statistically significant one, of lower lung-cancer mortality among men age 50 to 54 years who underwent low dose CT screening compared to those who underwent no screening. However, the NELSON study reported a statistically significant 26% reduction in lung cancer mortality in men of all ages (risk ratio = 0.74, 95% CI: 0.60–0.91) (de Koning 2020). Additionally, for the overall results from the NELSON study, the cumulative rate ratio for death from lung cancer at 10 years was 0.76 (95% confidence interval [CI], 0.61 to 0.94; P = 0.01) in the screening group as compared with the control group (de Koning, 2020). The authors concluded that for the overall results in the NELSON trial “involving high-risk persons, lung-cancer mortality was significantly lower among those who underwent volume CT screening than among those who underwent no screening” (de Koning, 2020).
The limitations in the subgroup analysis of men aged 50 to 54 years in the NELSON trial are that there was a small number of outcome events, such that 25 persons died of lung cancer in the screening arm compared to 31 persons in the control arm. Changes in these numbers can lead to a wide variation in the effect size under investigation in the clinical trial, which is the effect of low dose CT screening on lung cancer deaths. Additionally, the confidence interval (95% confidence interval [CI], 0.48 to 1.50) for the rate ratio of 0.85 appears to be wide and includes 1 or null effect, indicating a small sample size, a p-value greater than 0.05, and less confidence in this estimate of the true rate ratio. Moreover, the small number of events means that the likelihood of detecting any effect size by the clinical trial would probably be small, and as a result, a larger sample size would be needed to detect a small effect size, meaning that a large sample size would be needed to detect a small change in lung cancer deaths due to the effect of low dose CT screening on lung cancer deaths when compared to no screening. Additionally, the NELSON study authors reported that “the NELSON trial was not powered to show a possible favorable difference in all-cause mortality (expected within the range of 2.5%), because it would have required unrealistic sample sizes” (de Koning, 2020). Given the small number of lung cancer deaths, a small effect size, and wide confidence intervals, the NELSON study was likely underpowered for the subgroup analysis among age 50 to 54. The sample size was likely too small to show a difference in lung cancer mortality for those who had low dose CT screening compared to those with no screening. Thus, the NELSON study results suggest a clinically significant trend of decreasing lung cancer deaths, though not statistically significant, among men 50- to 54- years-old who received low dose CT lung cancer screening compared to those who had no screening. In contrast, the overall results from the NELSON study had a statistically significant cumulative lowering in lung cancer deaths among all men and women in the study.
While the main study population for the NELSON study (de Koning, 2020) consisted of men, two studies, the NELSON and the LUSI studies, had evidence for women on the effect of low dose CT screening on lung cancer mortality. In the NELSON study, women consisted of 16.7% (1,317/7,900) of the screening group (de Koning, 2020). However, the age distribution of the women in the screening group was not shown in the study results, so it is not possible to determine how many women in the screening group were aged 50- to 54-years-old though this age group of women was included in the NELSON study. Among women in the NELSON study, the rate ratio was 0.67 (95% CI, 0.38 to 1.14) at 10 years of follow-up indicating no observed difference in lung cancer mortality comparing those women who underwent LDCT to those who had no screening (de Koning, 2020). The NELSON study did not have any age-stratified results for the women in the study population. Thus, there was a strong trend, though not a statistically significant one, of lung-cancer mortality being 33% lower among women who underwent low dose CT screening compared to those who underwent no screening.
Similar to the subgroup age-stratified analysis for men, it is likely that the NELSON study was underpowered to find an effect of low dose CT screening on lung cancer mortality among women. There was a small number of outcome events, such that 25 women died of lung cancer in the screening arm compared to 36 persons in the control arm. Changes in these numbers can lead to a wide variation in the effect size under investigation in the clinical trial, which is the effect of low dose CT screening on lung cancer deaths. Additionally, the confidence interval (95% confidence interval [CI], 0.38 to 1.14) for the rate ratio of 0.67 appears to be wide and includes 1 or null effect, indicating a small sample size, a p-value greater than 0.05, and less confidence in this estimate of the true rate ratio. The small number of events means that the likelihood of detecting any effect size by the clinical trial would probably be small, thus a larger sample size would be needed to detect a small effect size, meaning that a large sample size would be needed to detect a small change in lung cancer deaths due to the effect of low dose CT screening on lung cancer deaths when compared to no screening. The NELSON study authors concluded that “trial data suggest greater benefits in women than in men, but in a subgroup with a relatively low number of women. More research is required in women, as well as in other subgroups” (de Koning, 2020).
While the main study population for the NELSON study (de Koning, 2020) consisted of men and had a subsample of women, the LUSI study (Becker, 2020) had a large enough study population for a stratified analysis specific to women. The German Lung cancer Screening Intervention (LUSI) was a randomized trial among 4,052 long-term smokers 50–69 years of age, recruited from the general population, comparing five annual rounds of low dose CT screening (screening arm; n = 2,029 participants) with a control arm without screening who received usual care (n = 2,023) (Becker, 2020). In the intervention arm, 49.9% (714/2,029) were women and there were 942 men and women age 50-to 54 years (46.4%; 942/2029; Becker, 2020). However, there were no age distribution categories for women in the study results, so it is not known how many women in the study were age 50 to 54 years though they were part of the inclusion criteria for age. Among the lung cancer deaths, 4 women died of lung cancer in the low dose CT arm compared to 13 women in the control arm. “Over an average observation time of 8.8 years after randomization, the hazard ratio [HR] for lung cancer mortality was 0.74 (95% CI: 0.46–1.19; p = 0.21) among men and women combined. Modeling by sex, however showed a statistically significant reduction in lung cancer mortality among women (HR = 0.31 [95% CI: 0.10–0.96], p = 0.04), but not among men (HR = 0.94 [95% CI: 0.54–1.61], p = 0.81) screened by LDCT”, and when testing for interaction by gender, “this heterogeneity was close to statistical significance (Pheterogeneity = 0.09)” (Becker, 2020). The results of the LUSI study showed a significant benefit for lung-cancer mortality in the small subgroup of women across all age groups between the ages of 50 and 69 years who were invited to undergo screening. The authors concluded that the “an intriguing observation in LUSI is the apparent heterogeneity (although only borderline significant, Pheterogeneity = 0.09) in the effect of LDCT screening on lung cancer mortality by sex, suggesting a mortality reduction among the women only,” and go on to report that the “findings from LUSI are in line with those from other trials, including NLST [National Lung Screening Trial], that suggest a stronger reduction of lung cancer mortality after LDCT screening among women as compared to men” (Becker, 2020).
The LUSI study authors report that this gender “heterogeneity could be the result of different relative counts of lung tumor [histologic] subtypes occurring in men and women,” but that the “numbers of cancer deaths in LUSI were too small to examine whether the apparent heterogeneity in relative mortality hazards for men and women could be explained by differences in tumor histology, or whether it could have been entirely due to chance” (Becker, 2020).
Two small European studies of low dose CT, the MILD and DLCST studies, included persons 49 to 54 years in its study population. The Multicentric Italian Lung Detection (MILD) trial prospectively randomized 4,099 participants aged from 49 to 75 years, to a screening arm (n = 2,376), with further randomization to annual (low dose CT every 12 months, n = 1,190) or biennial (low dose CT every 24 months, n = 1,186) low dose CT for a median period of 6 years, or control arm (n = 1,723) without intervention (Pastorino, Silva; Prolonged, July, 2019). In the intervention arm, 32.5% (773/2,376) were below age 55 years and 31.6% (750/2,376) were women (Pastorino, Silva; Prolonged, July, 2019).
However, there were no data that showed the number of women or men in the 50 to 54-year-old category. The low dose CT arm showed a statistically significant 39% reduced risk of lung cancer mortality at 10 years (adjusted hazard ratio [HR] 0.61; 95% confidence interval [CI] 0.39–0.95; p = 0.02) compared with the control arm for all men and women included in the MILD study (Pastorino, Silva; Prolonged, July, 2019). The Cox model analysis was adjusted for gender, age, and pack-years. However, there were no stratified analyses by specific age subgroup, such as age 50 to 54 years, or gender category for lung cancer mortality. The authors concluded that “the long-term results of the MILD trial show a statistically significant and clinically relevant 39% reduction of LC mortality at 10 years in the LDCT arm” (Pastorino, Silva; Prolonged, July, 2019). However, no data was shown on the magnitude of the statistically significant reduction in lung cancer deaths among men and women age 50- to 54-years-old even though that age group consisted of 32.5% of the study population.
The Danish Lung Cancer Screening Trial (DLCST) was a prospective, randomized screening trial comparing annual low-dose CT screening with no screening that enrolled a total of 4,104 men and women aged 50–70 years (Wille, 2016). The mean age of the screening group at randomization was 57.9 ± standard deviation of 4.8 and 56% were men (Wille, 2016). The frequency of persons in the 50-to 54-year-old age group was not shown, though the lower limit of the inclusion criteria included persons equal to or greater than 50 years-old. No differences between the two groups in lung cancer mortality (hazard ratio, 1.03; 95% confidence interval, 0.66–1.6; P = 0.888) with a mean of 9.47 person-years of follow up was observed (Wille, 2016). No age specific stratified subgroup analyses for lung cancer mortality were shown. For study limitations, the authors reported that the “reason that we did not see a difference in mortality may be small numbers; however, it may also be due to differences in inclusion criteria. The risk of lung cancer was probably lower in the DLCST cohort because participants were younger (50–70 years of age), had smoked less (lower limit was 20 pack-years), …” (Wille, 2016).
Additionally, the authors reported that “the annual incidence of lung cancer in our control group was only half what we expected (i.e., 0.27% instead of 0.50%); thus, the study was underpowered,” and concluded that “the limited statistical power of this trial does not allow a conclusive statement about the efficacy of lung cancer screening” but go on to say that “nevertheless, our results support risk stratification with a focus on age, smoking history, and obstructive lung disease when selecting candidates for lung cancer screening” (Wille, 2016). Thus, the DLCST study (Wille, 2016) did not show age-specific subgroup data on the effect of low dose CT screening on lung cancer deaths among 50- to 54-year-old persons, even though the inclusion criteria for age included persons greater than or equal to age 50 years, and the DLCST study results did not show a statistically significant lowering of lung cancer mortality in the low dose CT group.
Even though two small European studies included persons age 50- to 54-years-old, neither the UKLS nor the COSMOS study had results for lung cancer mortality. The United Kingdom Lung Cancer Screening pilot study (UKLS) trial by Field et al., (May, 2016) was a pilot randomized controlled trial comparing a single thoracic low dose CT scan intervention with usual care that randomized individuals aged 50–75 years (n = 4,055, with 4,061 enrolled and 6 not randomized) at high risk of lung cancer. The mean age of the screened arm was 67.1 ± 4.1 years. For the study population (n = 4,061 enrolled with 6 not randomized), 29 (0.7%) were age 50 to 55 years. A total of 1994 participants underwent CT scanning: 42 participants (2.1%) were diagnosed with lung cancer. The mean age at trial recruitment for the cancer patients was 66.9 years (median 67 years; range 55–75 years). No lung cancer mortality data were presented, and there were no age-stratified analyses for those diagnosed with lung cancer. The authors reported that “the UKLS pilot is insufficiently powered to demonstrate a reduction in mortality” (Field; May, 2016). The study authors concluded that “[l]ung cancer has been detected in 2.1% of people screened” and that “the specific successes include the demonstration that a population-based lung cancer screening trial can be undertaken in the UK, using a risk-based prediction model to select participants” (Field; May, 2016).
The aim of the Rampinelli (2017) study was to evaluate the cumulative radiation exposure and lifetime attributable risk of cancer incidence associated with low dose CT in a retrospective secondary analysis of data from a 10-year non-randomized observational lung cancer screening program, the Continuous Observation of Smoking Subjects (COSMOS) study. The single center COSMOS study in Italy enrolled high risk asymptomatic smokers aged 50 and older, who were current or former smokers (≥20 pack-years). The 50- to 54-year-old age group consisted of 34.4% (606/1759) women. The retrospective study included stratified analyses for the 50- to 54-year-old age group on the lifetime attributable risk of lung cancer and major cancers after 10 years of CT screening, but the COSMOS study by Rampinelli et al., (2017) did not have results for lung cancer-specific mortality data. Neither the UKLS nor the COSMOS study had results for lung cancer mortality. Thus, neither the COSMOS (Rampinelli, 2017) nor the UKLS (Field; May, 2016) studies had results for lung cancer mortality, even though persons age 50- to 54- years-old were included in the study populations.
Several low dose CT studies, such as the NLST, did not include persons age 50- to 54-years-old at enrollment into the study. For the extended follow up analysis of the high quality NLST trial, one of the two largest randomized controlled clinical trials of low dose CT, with the other being the NELSON study, the inclusion criterion for age was 55 to 74 years, so the lower age criterion was 55 years (NLST Research Team, 2019). The lowest age group at randomization was the 55- to 59-year-olds which was 42.8% (11,442/26,722) of the low dose CT intervention arm in the NLST trial (NLST Research Team, 2019). Persons age 50 to 54 years were excluded from the NLST study.
For the ITALUNG study, one of several small European randomized controlled clinical trials of low dose CT that excluded persons age 50- to 54-years-old, the inclusion criterion for age was 55 to 69 years (Paci, 2017), so the lower age limit was 55 years. In the active group, the mean age at entry was 60.9 years with the lowest age group of < 55 years comprising 3% (53/1.613) of the LDCT study population (Paci, 2017). Persons who were 50 to 54 years were not included in the ITALUNG study. For the small European DANTE study, the eligibility criterion for age was 60 to 74 years (Infante, 2015). The lower limit of the age criterion was 60 years, which excludes persons 50 to 54
years. In the low dose CT arm, the median age was 64.0 years (interquartile range: 5 years) and the mean age was 64.6 years (95% CI: 64.3-64.8) (Infante, 2015). The DANTE study results did not show the baseline age distribution for the study population stratified by age group, such as 50 to 54 years. The lung cancer mortality analyses of the DANTE study were not stratified by age group, such as 50 to 54 years. The DANTE study excluded persons 50- to 54-years-old. For the small LSS feasibility study (n = 3,318) conducted in the United States, the eligibility criterion for age was 55 to 74 years (Doroudi, 2018). With the lower limit of the age criterion being 55 years, the LSS study excluded persons 50 to 54 years.
Overall, based primarily on the NELSON, LUSI, and MILD study results, we find the human clinical evidence is sufficient to determine that screening for lung cancer with low dose CT is reasonable and necessary for the prevention or early detection of illness or disability for the population of 50- to 54-years-olds who are eligible for low dose CT. Given the limitations of the three studies, the overall evidence from the stratified results of the LUSI study and the overall results of the MILD and NELSON studies suggest a clinically significant lowering of lung cancer deaths among persons who were age 50 to 54 years who received low dose CT lung cancer screening compared to those who had no screening. The evidence suggests that lowering the starting age for low dose CT screening from 55 years to 50 years will continue to show benefit by reducing lung cancer deaths for women and very likely for men. The study results show sufficient evidence to lower the starting age for lung cancer screening with low dose CT. The evidence is sufficient for broadening the age eligibility criteria for lung cancer screening with low dose CT. Thus, with these limitations, we find that the evidence is sufficient to conclude that this approach to broadening the recommended population for lung cancer screening is reasonable and necessary for the prevention or early detection of illness or disability among Medicare beneficiaries with the specific eligibility criterion for starting age of screening set at 50 years.
The 2021 USPSTF recommendation on lung cancer screening now recommends that people start screening at age 50 (USPSTF, Krist; 2021), rather than age 55 years. As reported by the authors of the 2021 USPSTF evidence review by Jonas et al. (2021), “NLST and NELSON results are generally applicable to high-risk current and former smokers aged 50 to 74 years, but participants were younger, more highly educated, less likely to be current smokers than the US screening-eligible population, and had limited racial and ethnic diversity. The general US population eligible for lung cancer screening may be less likely to benefit from early detection compared with NLST and NELSON participants because they face a high risk of death from competing causes, such as heart disease and stroke. Data from the 2012 Health and Retirement Study showed a lower 5-year survival rate and life expectancy in screening-eligible persons compared with NLST participants” (Jonas, 2021). Consistent with the USPSTF grade B recommendation, our recommended screening population for starting age allows lung cancer screening to be reasonable and necessary for Medicare beneficiaries.
Among the societal organizations making recommendations for the selection of individuals for lung cancer screening, the American College of Chest Physicians (CHEST) has two recommendations. “1. For asymptomatic individuals age 55 to 77 who have smoked 30 pack- years or more and either continue to smoke or have quit within the past 15 years, we recommend that annual screening with low-dose CT should be offered. (Strong recommendation, moderate-quality evidence). 2. For asymptomatic individuals who do not meet the smoking and/or age criteria in Recommendation #1, are age 50 to 80, have smoked 20 pack-years or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with low-dose CT should be offered. (Weak recommendation, moderate-quality evidence)” (Mazzone, Panel Report 2021). Thus, for the lower age limit for starting low dose CT screening between 50 and 54 years, the American College of Chest Physicians guidelines only suggest low dose CT since this is a weak recommendation, and so it is not recommended since it is not a strong
recommendation.
In the updated National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Lung Cancer Screening Version 1.2022, the NCCN Lung Cancer Screening Panel defined the risk status of “high risk” as “age ≥ 50 years and ≥ 20 pack-year history of smoking” with these high-risk persons candidates for low dose CT (LDCT) screening (category 1) where “shared patient/provider decision-making is recommended, including a discussion of benefits/risks” (Wood, NCCN; 2021). For NCCN categories of evidence and consensus, Category 1 is defined as “based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate” (Wood, NCCN; 2021). Thus, the updated NCCN guidelines recommend starting screening at age 50 years.
In summary of their recommendations, the American College of Radiology Appropriateness Criteria expert panel concluded that for “Variant 1: Lung cancer screening with low-dose CT chest without IV contrast is usually appropriate in patients 55 to 80 years of age and 30 or more packs per year smoking history and currently smoke or have quit within the past 15 years. Variant 2: The panel did not agree on recommending lung cancer screening with low dose CT chest without IV contrast in patients 50 years of age or older and 20 or more packs per year history of smoking plus one additional risk factor. There is insufficient medical literature to conclude whether or not these patients would benefit from CT screening for lung cancer. Screening in this patient population is controversial but may be appropriate. Variant 3: Lung cancer screening is usually not appropriate in patients younger than 50 years of age or older than 80 years of age or in patients of any age with less than 20 packs per year history of smoking and no additional risk factors” (Donnelly, 2018). Thus, the American College of Radiology recommends starting screening at age 55 years.
The American Cancer Society 2013 “guideline recommends that clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30 [or more]–pack-year smoking history and who currently smoke or have quit within the past 15 years” (Wender, 2013). This American Cancer Society guideline was published around the time of the previous 2013 USPSTF lung cancer screening recommendation statement. Thus, the American Cancer Society recommends starting screening at age 55 years. However, the 2013 American Cancer Society guideline is being updated and, in the meantime, the American Cancer Society recommends yearly lung cancer screening with low dose CT scans for people who: are 50 to 80 years old and in fairly good health, currently smoke or have quit in the past 15 years, and have at least a 20 pack-year smoking history.
The American Academy of Family Physicians gives the clinical preventive services recommendation for lung cancer screening in adults a B grade recommendation. “The AAFP supports the United States Preventive Services Task Force (USPSTF) recommendation for annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years” (AAFP, 2021). Thus, the American Academy of Family Physicians recommends starting screening at age 50 years.
We find that the human clinical evidence is sufficient to conclude that lung cancer screening with low dose CT is reasonable and necessary for prevention or early detection of illness or disability, for Medicare beneficiaries with the specific eligibility criterion for starting low dose CT screening at age 50 years. This approach to broadening the starting age for lung cancer screening from 55 to 50 years is consistent with the USPSTF grade B recommendation.
Changing Stopping Age From 77 years to 80 years
The current 2021 USPSTF lung cancer screening statement recommends a stopping age for screening of 80 years (USPSTF, Krist; 2021). This stopping age is the same as the 2013 USPSTF recommendation of stopping screening at age 80 years (Moyer, USPSTF; 2014). However, the 2015 NCD on Screening for Lung Cancer with LDCT had the stopping age for screening at 77 years because there were no trial data or evidence on adults over 77 years (CAG-00439N; CMS, 2015). For this reconsideration, we will assess the recently updated trial data published from 2014 to the present for evidence of the effect of screening with low dose CT among persons age 77 years to 80 years.
Many low dose CT studies did not include persons age 77- to 80-years-old at time of enrollment into the study. For the extended follow up analysis of the NLST trial, the inclusion criterion for age was 55 to 74 years, so the upper age criterion was 74 years (NLST Research Team, 2019). The upper age group at randomization was 70- to 74-years-old and consisted of 8.8% (2,354/26,722) of the low dose CT screening arm in the NLST trial (NLST Research Team, 2019). Persons age 74 to 80 years were excluded from the NLST study.
For the NELSON study, the inclusion criterion for age was 50 to 74 years (de Koning, 2020). At randomization, the median age of the male participants was 58 years in the low dose CT screening group (interquartile range, 55 to 63 in the screening group) and while the highest age group of > 75
years comprised 0.6% (40/6,560) of the screening group, the age range for men was from 46 years to 76 years (de Koning, 2020). The NELSON study did not include persons 77- to 80-years-old.
For the ITALUNG study, one of several small European randomized controlled clinical trials of low dose CT, the inclusion criterion for age was 55 to 69 years (Paci, 2017), so the upper age limit was 69 years. In the active group, the mean age at entry was 60.9 years with the uppermost age group of > 69 years comprising 0.3% (5/1,613) of the LDCT study population (Paci, 2017). Persons who were 70- to 80-years-old were not included in the ITALUNG study. The inclusion criterion for age in the LUSI study was 50 to 69 years (Becker, 2020). The median age in the intervention arm of the LUSI study was 55 years and the upper age group 65 to 69 years comprised 11.1% (225/2,029) of the intervention arm (Becker, 2020). The LUSI study did not include persons 70- to 80-years-old. For the MILD study, the inclusion criterion for age was 49 to 75 years (Pastorino, Silva; Prolonged, July, 2019). The median age of the intervention arm was 58 years and the upper age group of ≥ 70 years comprised 3.8% (90/2,376) of the intervention arm in the MILD study (Pastorino, Silva; Prolonged, July, 2019). The MILD study excluded persons 76- to 80-years-old.
For the DLCST study, the inclusion criterion for age was 50 to 70 years (Wille, 2016). The mean age of the screening group at randomization was 57.9 ± standard deviation of 4.8 (Wille, 2016). The upper limit of the inclusion criteria included persons equal to or less than 70 years-old. Thus, the DLCST study did not include persons 71 to 80 years-old. For the UKLS study, the inclusion criterion for age was 50–75 years (n = 4,055, with 4,061 enrolled and 6 not randomized) at high risk of lung cancer (Field; May, 2016). The uppermost age group was 71- to 75-years-old (Field; May, 2016). For the study population (n = 4,061 enrolled with 6 not randomized), 22.4% were age 71 to 75 years and the mean age of the screened arm was 67.1 ± 4.1 years (Field; May, 2016). Thus, the UKLS study excluded persons 76- to 80-years-old.
For the small European DANTE study, the eligibility criterion for age was 60 to 74 years (Infante, 2015). The upper limit of the age criterion was 74 years, which excludes persons 77 to 80 years. In the low dose CT arm, the median age was 64.0 years (interquartile range: 5 years) and the mean age was 64.6 years (95% CI: 64.3-64.8) (Infante, 2015). The DANTE study excluded persons 75- to 80-years-old. The retrospective analysis of the small COSMOS study enrolled high risk asymptomatic smokers aged 50 and older, who were current or former smokers (≥20 pack-years) (Rampinelli, 2017). For the COSMOS study, the ≥ 65 years age group comprised 12.6% (658/5203) of the trial participants (Rampinelli, 2017). The COSMOS study does not appear to have persons 77- to 80-years-old in the study population. For the small LSS feasibility study (n = 3,318) conducted in the United States, the eligibility criterion for age was 55 to 74 years (Doroudi, 2018). With the upper limit of the age criterion being 74 years, the LSS study excluded persons 75 to 80 years.
Thus, none of the ten randomized controlled clinical trials, including the COSMOS, DANTE, DLCST, ITALUNG, LSS, LUSI, MILD,
NELSON, NLST, and UKLS studies, had persons age 77- to 80-years-old in the study populations.
In our literature search, one observational study, study by Liang et al., (2019 included individuals in age categories beyond 77 years, categorized as 75 to 79 years, 80 to 84 years, and greater than or equal to 85 years. The Liang et al., (2019) study used official cancer registry data from Shanghai municipality and its two districts with different levels of accessibility for low dose CT, defined as urban versus suburban access, to assess the potential effect of low dose CT on lung cancer incidence and mortality, but the study had no patient-level data on low dose CT. A major limitation reported by the study authors was that they could not “determine which patients were diagnosed through LDCT screening, or how many people were screened in the different regions” (Liang, 2019). In addition, the Liang (2019) study did not follow individuals over time and had no prospective follow up information on outcomes such as lung cancer mortality. As a result, the study did not calculate lung cancer mortality as a hazard ratio or a rate ratio using a survival function. Thus, the Liang (2019) cancer registry study, while including persons beyond 77 years-old, was excluded from the analysis because it was not a prospective randomized controlled trial of using low dose CT to screen for lung cancer and it did not have information on whether an individual was screened with low dose CT.
Our findings on the lack of evidence on adults over 77 years are consistent with our 2015 decision memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N; CMS, 2015).
Overall, there are no lung cancer screening trial data or evidence on adults over 77 years. There is insufficient evidence to determine if these patients over 77 years would benefit from low dose CT screening for lung cancer. There is no relevant published human clinical study literature regarding the use of low dose CT in persons age 77- to 80-years-old. We find the human clinical evidence is insufficient to determine that screening for lung cancer with low dose CT is reasonable and necessary for the prevention or early detection of illness or disability for the population of 77- to 80-years-old who are eligible for low dose CT. The study results do not show sufficient evidence to change the eligibility criterion for the age to stop lung cancer screening with low dose CT. Thus, we find that the evidence is not sufficient to conclude that lung cancer screening is reasonable and necessary for Medicare beneficiaries with the specific eligibility criterion for the stopping age of 80 years. The Medicare beneficiary eligibility criterion for stopping age will not be changed and will remain at 77 years-old.
Among the organizations making recommendations for the selection of individuals for lung cancer screening, the American College of Chest Physicians (CHEST) has two recommendations. “1. For asymptomatic individuals age 55 to 77 who have smoked 30 pack- years or more and either continue to smoke or have quit within the past 15 years, we recommend that annual screening with low-dose CT should be offered. (Strong recommendation, moderate-quality evidence). 2. For asymptomatic individuals who do not meet the smoking and/or age criteria in Recommendation #1, are age 50 to 80, have smoked 20 pack-years or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with low-dose CT should be offered. (Weak recommendation, moderate-quality evidence)” (Mazzone, Panel Report 2021). In the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), they presented no data on the effect of screening cessation between age 77 and 80 years on lung cancer mortality. The CHEST guidelines meta-analysis stops the age at screening cessation at 75 years and has no data beyond the stopping age of 76 or more years.
In the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Lung Cancer Screening Version 1.2022, the NCCN Lung Cancer Screening Panel defined the risk status of “high risk” as “age ≥ 50 years and ≥ 20 pack-year history of smoking” with these high-risk persons candidates for low dose CT (LDCT) screening (category 1) where “shared patient/provider decision-making is recommended, including a discussion of benefits/risks” (Wood, NCCN; 2021). Further, in the discussion of extending low dose CT screening beyond 77 years that is being updated in the most recent NCCN guidelines Version 1. 2022 (Wood, NCCN; 2021), no randomized controlled trial data were presented that showed whether low dose CT screening in persons age 77 or more years had an effect on lung cancer mortality, and so the evidence base for extending the eligibility criterion for low dose CT screening beyond 77 years does not appear to have randomized controlled trial evidence. Thus, in the updated National Comprehensive Cancer Network (NCCN) guidelines Version 1.2022, the eligibility criterion for age has no upper age limit in the low dose CT screening algorithm, and therefore, does not include a stopping age for low dose CT screening.
In summary of their recommendations, the American College of Radiology Appropriateness Criteria expert panel concluded that for “Variant 1: Lung cancer screening with low-dose CT chest without IV contrast is usually appropriate in patients 55 to 80 years of age and 30 or more packs per year smoking history and currently smoke or have quit within the past 15 years. Variant 2: The panel did not agree on recommending lung cancer screening with low dose CT chest without IV contrast in patients 50 years of age or older and 20 or more packs per year history of smoking plus one additional risk factor. There is insufficient medical literature to conclude whether or not these patients would benefit from CT screening for lung cancer. Screening in this patient population is controversial but may be appropriate. Variant 3: Lung cancer screening is usually not appropriate in patients younger than 50 years of age or older than 80 years of age or in patients of any age with less than 20 packs per year history of smoking and no additional risk factors” (Donnelly, 2018). Thus, the American College of Radiology recommends stopping screening at age 80 years.
Similarly, the American Academy of Family Physicians gives the clinical preventive services recommendation for lung cancer screening in adults a B grade recommendation. “The AAFP supports the United States Preventive Services Task Force (USPSTF) recommendation for annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years” (AAFP, 2021). Thus, the American Academy of Family Physicians recommends stopping screening at age 80 years.
The American Cancer Society 2013 “guideline recommends that clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30 [or more]–pack-year smoking history and who currently smoke or have quit within the past 15 years.” (Wender, 2013). However, the 2013 American Cancer Society guideline is being updated and, in the meantime, the American Cancer Society recommends yearly lung cancer screening with low dose CT scans for people who: are 50 to 80 years old and in fairly good health, currently smoke or have quit in the past 15 years, and have at least a 20 pack-year smoking history.
We find that the human clinical evidence is not sufficient to conclude that lung cancer screening with low dose CT is reasonable and necessary for Medicare beneficiaries with the specific eligibility criterion for stopping low dose CT screening at age 80 years. The Medicare beneficiary eligibility criterion for stopping age will not be changed and will remain at 77 years-old.
Lowering Pack-Year Smoking History From 30 Pack-Years to 20 Pack-Years
The 2021 USPSTF lung cancer screening statement recommends annual lung cancer screening with low dose CT for adults who have a 20 pack-year smoking history (USPSTF, Krist; 2021). The current updated 2021 USPSTF recommendation reduces the pack-years of smoking history that makes someone eligible for screening from 30 pack-years to 20 pack-years. Thus, the pack-year eligibility criterion was lowered from 30 pack-years in the previous 2013 USPSTF recommendation (Moyer, USPSTF; 2014) to 20 pack-year smoking history in the current updated recommendation. For this reconsideration, we will assess the recently updated trial data published from 2014 to the present for evidence on the beneficial effect of screening with low dose CT among persons who have a smoking history of 20 to 29 pack-years.
The MILD study (Pastorino, Silva, Sestini; 2019) defined the enrollment eligibility criterion for current smokers as ≥ 20 pack-years (Pastorino, Silva, Sestini; 2019). In the intervention arm, 21.9% (521/2,376) had < 30 pack-years of cigarette smoking and the median pack-years was 39 pack-years of smoking (Pastorino, 2019). Taking into account the minimum enrollment criterion for smokers of at least 20 pack-years, 21.9% of the intervention arm smoked 20 to 29 pack-years. In the MILD study, the low dose CT arm showed a significant 39% reduced risk of lung cancer mortality at 10 years compared to the control arm (hazard ratio = 0.61; 95% CI 0.39 – 0.95; p = 0.02) with adjustment for age and pack-years (Pastorino, Silva, Sestini; 2019). Even though there was no stratified lung cancer mortality analysis by smoking history subgroups in the number of pack-years, such as 20 to 29 pack-years of smoking cigarettes, the overall study results demonstrated statistically significant reduced lung cancer mortality, 39%, in the low dose CT arm which included persons who smoked between 20 to 29 pack-years with the large subgroup comprising 21.9% of the overall study population. Thus, there is evidence from the MILD study that lowering the smoking history from 30 pack-years to 20 pack-years would likely continue to reduce lung cancer deaths among persons who are screened for lung cancer with low dose CT.
In the good quality NELSON study, the eligibility inclusion criterion for smoking history was persons who had smoked >15 cigarettes a day for > 25 years or >10 cigarettes a day for >30 years (de Koning, 2020). In pack-years, >15 cigarettes a day for > 25 years is equivalent to > 19 pack-years, and >10 cigarettes a day for >30 years is equivalent to >15 pack-years. Persons who smoked 20 to 29 pack-years were included in the NELSON study. Among the male participants in the screening group for the NELSON study, the median pack-year of smoking was 38.0 (interquartile range: 29.7 – 49.5) with a range of pack-years from 0.4 to 159.5 (de Koning, 2020). The specific number of pack-years smoked by the male participants is not shown in the NELSON study results. However, the NELSON study reported on the number of cigarettes smoked per day and the duration of smoking in years for the male participants.
Because the number of pack-years smoked by participants in the NELSON study was not used to define smoking history, it is necessary to show how to convert the number of cigarettes smoked per day and the duration of smoking to pack-years smoked. Pack-years, or packs per years, are calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked, with a pack equal to 20 cigarettes. If a person has smoked a pack a day for the last 20 years, or two packs a day for the last 10 years, the person has a smoking history of 20 pack-years. Equivalently, if a person has smoked 20 cigarettes a day, which is one pack a day, for the last 20 years; or 40 cigarettes a day, which is two packs a day, for the last 10 years, the person has a smoking history of 20 pack-years.
Among the male participants in the screening group (n = 6,583) for the NELSON study, 28.3% (1,859/6,565) smoked 16 to 20 cigarettes a day, which is about one pack a day, and 0.4% (25/6,563) had a duration of smoking of ≤ 25 years which includes an unspecified number of persons smoking for the last 20 years which is below 0.4%, meaning that an undetermined but likely small but non zero number of men smoked about one pack per day for perhaps 20 years which approximates a 20 pack-year smoking history. Equivalently to a 20 pack-year history, 6.9% (454/6,565) smoked 31 to 40 cigarettes a day, which is about two packs a day, and 0.4% (25/6,563) had a duration of smoking of ≤ 25 years which includes an unspecified number of persons smoking for the last 10 years which is below 0.4% (de Koning, 2020). Because the NELSON study results did not include the pack-year smoking history stratified by the number of pack-years, it is not known exactly how many men had a smoking history of 20 to 29 pack-years. However, the NELSON study included men who smoked about one pack per day (28.3%) and about two packs per day (6.9%), and those men smoked for ≤ 25 years (0.4%) with some of them, less than 0.4%, likely smoking for the last 20 years at one pack per day or 10 years at two packs per day, which would amount to a 20 pack-year smoking history. In addition, the lower limit of the range of pack-years of smoking was 0.4, so the range of pack-years of smoking encompasses 20 to 29 pack-years. Thus, while the exact number of men smoking 20 to 29 pack-years is not known, men with a smoking history of 20 to 29 pack-years would very likely have been included in the NELSON study, though the precise number is likely to be small but not zero. Additionally, the eligibility criterion for smoking history permitted persons who smoked 20 to 29 pack-years to be included in the NELSON study.
For the NELSON study at 10 years of follow up among men, the cumulative rate ratio for death from lung cancer was significantly lower (rate ratio [RR] = 0.76, 95% CI: 0.61 to 0.94; p = 0.01) among those in the screening group who underwent low dose CT as compared with the control group who underwent no screening (de Koning, 2020). Among the small subsample of women, the non-significant rate ratio was 0.67 (95% CI: 0.38 to 1.14) at 10 years of follow up for lung cancer mortality comparing the low dose CT group to the control group, though the rate ratios were statistically significant at 7, 8, and 9 years of follow up (at 7 years: rate ratio = 0.46; 95% CI: 0.21 – 0.96; de Koning, 2020). There were no stratified analyses of lung cancer mortality by number of pack-years smoked as a subgroup that were shown in the NELSON study results. The authors concluded that “in this trial involving high-risk persons, lung cancer mortality was significantly lower among those who underwent volume CT screening than among those who underwent no screening” (de Koning, 2020). Additionally, the eligibility criterion for smoking history permitted persons who smoked 20 to 29 pack-years to be included in the NELSON study.
Because the NELSON study showed a statistically significantly lower lung cancer death rate among men of all ages who underwent low dose CT and included persons who smoked 20 to 29 pack-years of cigarettes, the NELSON study provides convincing evidence for the discussion of lowering the smoking history eligibility criterion.
Other small European studies, such as the LUSI and ITALUNG studies, had data on smokers with a 20 to 29 pack-year smoking history, but had differing results on lung cancer mortality. In the LUSI study, current smokers were described as “long-term smokers” but the study authors did not define “long-term smokers” (Becker, 2020). Eligibility for the LUSI study by smoking history criterion was defined by at least 25 years of smoking of at least 15 cigarettes per day, which is equivalent to at least 19 pack-years; or at least 30 years smoking of at least 10 cigarettes per day, which is at least equivalent to 15 pack-years (Becker, 2020). In the low dose CT intervention arm of the LUSI study, 50.2% were current smokers and 49.8% were ex-smokers (Becker, 2020). No definition of “current” smoker or “ex-smoker” was given in the study results. There were no data presented on the distribution of smoking history by subgroup of the number of pack-years smoked for the study population in the LUSI study results.
Over an average observation time of 8.8 years after randomization, “modeling by sex, however, showed a statistically significant reduction in lung cancer mortality among women (HR [hazard ratio] = 0.31 [95% CI: 0.10-0.96], p = 0.04), but not among men (HR = 0.94 [95% CI: 0.54-1.61], p = 0.81) screened by LDCT….. and this heterogeneity was close to statistical significance ([Pheterogeneity = 0.09)” (Becker, 2020). There were no stratified analyses by smoking history for number of pack-years of smoking for lung cancer mortality in the LUSI study results. Because the smoking history eligibility criterion was defined as having at least 15 or 19 pack-years of smoking, it appears that the study population for the LUSI trial is likely to include smokers with a 20 to 25 pack-year smoking history. Thus, the LUSI study showed that low dose CT screening among persons with a 20 to 29 pack-year history reduces lung cancer mortality, especially among women.
Several studies, the ITALUNG, the DANTE, and the DLCST studies, had data on smokers with a smoking history of at least 20 pack-years but did not show a statistically significant effect on lung cancer mortality. The ITALUNG study, one of several small European randomized controlled clinical trials of low dose CT, randomized eligible persons to receive an annual invitation for low dose CT screening for four years, the active group (n = 1,613), or to usual care, the control group, in three screening centers in Italy (Paci, 2017). The ITALUNG study (Paci, 2017) defined the enrollment eligibility criterion for smokers as a smoking history of at least 20 pack-years in the last 10 years (Paci, 2017). Persons with a 20 to 29 pack-year history of smoking were included in the ITALUNG study. In the active group that received low dose CT, the median pack-years of smoking was 40 pack-years. The active group consisted of 66% current smokers (Paci, 2017). There was no data shown on the distribution of the number of pack-years smoked in the ITALUNG study results. After a median follow up of 9.3 years, study results showed a non-significant reduction of 30% (RR = 0.70; 95% CI 0.47 to
1.03; p = 0.07) for lung cancer mortality in the active LDCT group compared to the usual care control group (Paci, 2017). However, the study authors reported that “none of these published outcome studies alone (DANTE, MILD, and DLCST), including the ITALUNG, has sufficient statistical power to detect a real benefit and, for specific analyses, as those assessing benefit for risk profile subgroups” (Paci, 2017). In the ITALUNG study results, there were no stratified analyses by pack-year smoking history for lung cancer mortality. While the ITALUNG study included smokers with a smoking history of at least 20 pack-years, the results showed a non-statistically significant reduction for lung cancer mortality in the low dose CT intervention group compared to the control group.
The DANTE study (Infante, 2015) defined the enrollment eligibility criterion for smokers of at least 20 pack-years, defined as “20+ pack-years” (Infante, 2015). Persons with a 20 to 29 pack-year history of smoking were included in the DANTE study. In the low dose CT group, the mean pack-years was 47.3 (95% confidence interval 45.7 - 49.0) with a standard error of the mean of 0.8, and a median pack-years of 45.0 (interquartile range 28.5) (Infante, 2015). There was no stratified distribution of smoking history by number of pack-years subgroups shown in the DANTE baseline study results. For lung cancer mortality, the hazard ratio was 0.993 with a 95% CI of 0.688 to 1.433 (Infante, 2015). Lung cancer mortality did not differ significantly between the two arms of LDCT screening compared to annual clinical review (Infante, 2015). There were no stratified analyses of lung cancer mortality by pack-years of smoking history subgroup shown in the DANTE study results. The authors concluded that “given the limited statistical power of the DANTE [Detection And screening of early lung cancer with Novel imaging TEchnology] trial, our data do not allow making a definitive statement about whether or not LDCT screening is effective in reducing lung cancer mortality” (Infante, 2015). While the DANTE study included smokers with a smoking history of at least 20 pack-years, the results showed a non-statistically significant reduction for lung cancer mortality in the low dose CT intervention group compared to the control group.
In the DLCST trial, the inclusion criterion for smoking history was a minimum of 20 pack-years of smoking (Wille, 2016). In the screening group (n = 2,052), the mean pack-years was 36.4 ± 13.4 pack-years (standard deviation) (Wille, 2016). In the DLCST study, no difference between the low dose CT study group and the no screening control group in lung cancer mortality (hazard ratio = 1.03; 95% CI: 0.66 – 1.6; p = 0.888; Wille, 2016) was observed. The study results for lung cancer mortality were not stratified by the number of pack-years smoked for the subgroup of 20 to 29 pack-years smoked. The authors of the DLCST trial concluded that “no statistically significant effects of CT screening on lung cancer mortality were found, but the results of post hoc high-risk subgroup analyses showed nonsignificant trends that seem to be in good agreement with the results of the National Lung Screening Trial [NLST]” (Wille, 2016). The study authors also reported that “the study was underpowered, and the results of the DLCST do not allow us to make definitive conclusions about the efficacy of screening” (Wille, 2016). While the DLCST study included persons with a 20+ pack-year history of smoking, the results did not show that low dose CT screening lowers lung cancer deaths more so than in a no screening group. However, the study authors reported that the DLCST study may be underpowered and have a sample size that is too small to show a significant difference in lung cancer mortality among those receiving LDCT compared to no screening.
Thus, the ITALUNG, the DANTE, and the DLCST studies had data on smokers with a smoking history of 20 to 29 pack-years, but the results did not show that low dose CT screening had a significant effect on lung cancer mortality.
One study, the COSMOS study, had data on smokers with a smoking history of 20 to 29 pack-years but the study results did not have data on lung cancer mortality. In the secondary analysis of the COSMOS study, the eligibility criterion for smoking history was ≥ 20 pack-years of smoking (Rampinelli, 2017). No data on lung cancer mortality was presented in the secondary analysis of the COSMOS trial study results. The secondary analysis of the COSMOS study did not contribute data to the evaluation of the pack-year smoking history eligibility criterion.
Several low-dose CT studies, such as the NLST, the LSS, and the UKLS, did not have data on persons who had a 20 to 29 pack-year history of smoking. For the extended follow up analysis of the good quality NLST (National Lung Screening Trial) trial, the pack-year smoking history criterion was a minimum of 30 pack-years of cigarette smoking (NLST Research Team, 2019). This eligibility criterion excluded persons with a 20 to 29 pack-year smoking history from enrolling in the NLST trial. For the low dose CT arm (n = 26,722), 48.1% were current smokers and the median pack-years was 48 (25th/75th percentile: 39/66) (NLST Research Team, 2019). In the NLST study results, no data was shown on the number of pack-years of cigarette smoking. “Current” smokers were not defined in the Methods of the NLST study. Because the NLST study does not have data on smokers with a 20 to 29 pack-year history of cigarette smoking, the NLST study results do not address the potential change in the pack-year smoking history eligibility criterion.
For the LSS study, the eligibility criterion for smoking history was “a 30 pack-year history of cigarette smoking” (Doroudi, 2018). There were no data shown on the number of pack-years smoked for the study population in the LSS study results (Doroudi, 2018). Because the LSS study did not include persons with a 20 to 29 pack-year smoking history, the LSS study results did not contribute evidence to the discussion of lowering the pack-year smoking history criterion. In the UKLS study, who were “current smokers” was not defined. Among the 75,958 positive responders to the invitation letter to participate in the UKLS trial, 14.7% were current smokers. Among the 2,028 participants in the screening arm, 38.3% were current smokers (Field; May, 2016). No data on the number of pack-years smoked by current smokers were shown in the UKLS study results. Combining current and ex-smokers, 93.4% had a smoking duration of 20+ years and 5.8% had a smoking duration of 10 - 19 years (Field; May, 2016). The UKLS study did not have data on lung cancer mortality (Field; May, 2016). None of the analyses were stratified by pack-years of smoking. Because the UKLS study did not have the number of pack-years smoking history, the UKLS study did not have data that would affect the Analysis of lowering the pack-years smoking history.
Overall, based primarily on the MILD, NELSON, and LUSI study results, we find the human clinical evidence is sufficient to determine that screening for lung cancer with low dose CT is reasonable and necessary for the prevention or early detection of illness or disability for the population of persons who have a smoking history of 20 to 29 pack-years who are eligible for low dose CT. Given the limitations of the three studies lacking stratified analyses, the evidence from the overall results of the MILD, NELSON, and LUSI studies suggest a clinically significant lowering of lung cancer deaths among persons who had a 20 to 29 pack-year history of smoking who received low dose CT lung cancer screening compared to those who had no low dose CT screening. The evidence supports that lowering the eligibility criterion for smoking history for low dose CT screening from 30 pack-years to 20 pack-years will continue to show benefit by reducing lung cancer deaths among persons eligible for low dose CT screening. The study results show sufficient evidence to lower the smoking history criterion which would expand the population eligible for lung cancer screening with low dose CT. Thus, with these limitations, we find that the evidence is sufficient to conclude that this approach to broadening the recommended population for lung cancer screening is reasonable and necessary for the prevention or early detection of illness or disability among Medicare beneficiaries with the specific eligibility criterion for a smoking history of at least a 20 pack-years.
In the 2021 USPSTF recommendation statement on lung cancer screening, “[t]he USPSTF recommends annual screening for lung cancer with LDCT in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (USPSTF, Krist; 2021). The 2021 USPSTF lung cancer screening statement recommends annual lung cancer screening with low-dose CT for adults who have a 20 pack-year smoking history and currently smoke (USPSTF, Krist; 2021). This revised National Coverage Determination policy that lowers the smoking pack-year history eligibility criterion aligns with the 2021 USPSTF recommendation statement on pack-years smoking history.
Among the societal organizations making recommendations for the selection of individuals for lung cancer screening, the American College of Chest Physicians (CHEST) has two recommendations. “1. For asymptomatic individuals age 55 to 77 who have smoked 30 pack- years or more and either continue to smoke or have quit within the past 15 years, we recommend that annual screening with low-dose CT should be offered. (Strong recommendation, moderate-quality evidence). 2. For asymptomatic individuals who do not meet the smoking and/or age criteria in Recommendation #1, are age 50 to 80, have smoked 20 pack-years or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with low-dose CT should be offered. (Weak recommendation, moderate-quality evidence)” (Mazzone, Panel Report 2021). Thus, for the smoking history for persons who smoked 20 to 29 pack-years, the American College of Chest Physicians guidelines only suggest low dose CT since this is a weak recommendation, and so it is not recommended since it is not a strong recommendation.
In the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Lung Cancer Screening Version 1.2021, the NCCN Lung Cancer Screening Panel defined the risk status of “high risk” as “age ≥ 50 years and ≥ 20 pack-year history of smoking” with these high-risk candidates for low dose CT (LDCT) screening (category 1) where “shared patient/physician decision-making is recommended, including a discussion of benefits/risks” (NCCN, 2020). The updates in the clinical pathway algorithms in the current Version 1.2021 (NCCN, 2020) of the NCCN Guidelines for Lung Cancer Screening from Version 1.2020 include high risk status being modified with the age range changed from 55 to 77 years to only ≥ 50 years and lowered from ≥ 30 to ≥ 20 pack-year history of smoking (NCCN, 2020). Regarding lowering the pack-year smoking history criterion, the current NCCN guidelines Version 1.2021 reported that “data suggest that the lung cancer risk for individuals with a 20 to 29 pack-year smoking history is similar to that of individuals with a 30 or more pack-year history” (NCCN, 2020) which supports the current NCCN recommendation of low dose CT screening among persons with a 20 or more pack-year smoking history. The pack-year history criterion for high risk individuals remains unchanged in the updated NCCN recommendation (Wood, NCCN, 2021).
Similarly, the American Academy of Family Physicians gives the clinical preventive services recommendation for lung cancer screening in adults a B grade recommendation. “The AAFP supports the United States Preventive Services Task Force (USPSTF) recommendation for annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery” (AAFP, 2021). Thus, the American Academy of Family Physicians recommends screening in adults who have a 20 pack-year
smoking history.
In summary of their recommendations, the American College of Radiology Appropriateness Criteria expert panel concluded that for “Variant 1: Lung cancer screening with low-dose CT chest without IV contrast is usually appropriate in patients 55 to 80 years of age and 30 or more packs per year smoking history and currently smoke or have quit within the past 15 years. Variant 2: The panel did not agree on recommending lung cancer screening with low dose CT chest without IV contrast in patients 50 years of age or older and 20 or more packs per year history of smoking plus one additional risk factor. There is insufficient medical literature to conclude whether or not these patients would benefit from CT screening for lung cancer. Screening in this patient population is controversial but may be appropriate. Variant 3: Lung cancer screening is usually not appropriate in patients younger than 50 years of age or older than 80 years of age or in patients of any age with less than 20 packs per year history of smoking and no additional risk factors” (Donnelly, 2018). Thus, the American College of Radiology could not agree on recommending lung cancer screening with low dose CT in patients with 20 or more packs per year history of smoking.
The American Cancer Society 2013 “guideline recommends that clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30 [or more]–pack-year smoking history and who currently smoke or have quit within the past 15 years.” (Wender, 2013). This American Cancer Society guideline was published around the time of the previous 2013 USPSTF lung cancer screening recommendation statement. Thus, the American Cancer Society recommends screening persons who have at least a 30 pack-year smoking history. However, the 2013 American Cancer Society guideline is being updated and, in the meantime, the American Cancer Society recommends yearly lung cancer screening with low dose CT scans for people who: are 50 to 80 years old and in fairly good health, currently smoke or have quit in the past 15 years, and have at least a 20 pack-year smoking.
We find that the empiric evidence is sufficient to conclude that lung cancer screening with low- dose CT is reasonable and necessary for the prevention or early detection of illness or disability, for Medicare beneficiaries with the specific eligibility criterion for a smoking history of 20 or more pack-years. This approach to broadening the smoking history from 30 pack-years to 20 or more pack-years is consistent with the USPSTF grade B recommendation.
Maintaining Quit Smoking History at Quitting Within Past 15 Years
In 2021, the USPSTF recommends annual lung cancer screening with low-dose CT in adults who have quit within the past 15 years (USPSTF, Krist; 2021). Similar to the 2013 USPSTF recommendation, screening should be discontinued once a person has not smoked for 15 years (Moyer, USPSTF; 2014). Thus, in both 2013 (Moyer, USPSTF; 2014) and 2021 (USPSTF, Krist; 2021), the USPSTF recommends stopping lung screening once a person has not smoked for 15 years. We received public comments during the initial public comment period requesting removal of the eligibility criterion for the quit smoking history. We decided to assess whether the clinical studies had data on quit smoking history for former smokers who quit within the past 15 years to confirm the effectiveness of the current recommendation. We also assessed whether the clinical studies had data on quit smoking history for former smokers who quit smoking for at least 15 years or more to determine whether lung cancer mortality is reduced among those who received low-dose CT and had quit smoking for more than 15 years. If the data show the effectiveness of low-dose CT screening by reducing lung cancer deaths among persons who quit smoking for more than 15 years, then it might be plausible to consider eliminating the quit smoking criterion of quitting for the past 15 years since low dose CT would still be effective in reducing lung cancer deaths among those who quit smoking beyond 15 years ago.
One study, the good quality NLST trial, included former smokers who had quit within the past 15 years, but excluded persons who had quit more than 15 years ago. For the extended follow up analysis of the NLST trial the quitting smoking history inclusion criterion was persons who had quit within the past 15 years (NLST, 2019) and so former smokers who had quit within the past 15 years, such as quitting 14 years ago, were included in the NLST trial. However, persons who had quit beyond 15 years
ago, such as someone who had quit 16 years ago, was not included in the NLST study. This eligibility criterion excludes persons who had quit more than 15 years ago. The NLST randomized high-risk current and former smokers to three annual screens with either low dose CT or chest radiography (CXR) in the United States (NLST, 2019). For the low dose CT arm (n = 26,722), 51.9% were former smokers (NLST Research Team, 2019). In the NLST study results, no data was shown on the number of years of
quitting smoking. The definition of “former” smokers was not provided in the publication.
After a median of 12.3 years of follow-up, the overall NLST study results demonstrated a statistically significant reduction in lung cancer mortality (dilution-adjusted lung cancer mortality rate ratio = 0.89; 95% CI: 0.80-0.997; p = 0.043; NLST Research Team, 2019) in the low dose CT arm, which is also clinically significant. The dilution-adjusted lung cancer mortality rate ratio is a more appropriate outcome measure to use than an overall lung cancer mortality rate ratio because “with follow-up now well beyond the period of trial screening, there is the potential, or even likelihood, of some dilution of the screening effect. Specifically, patients in whom cancer did not develop until after the last scheduled screen could not have benefited from the trial screenings; therefore, deaths in such patients would only serve to add noise to the estimates, roughly an equal number of deaths in each arm. Therefore, in analyzing these data, we employ various methods that attempt to control for a dilution effect, including examining the difference across arms in lung cancer deaths in addition to the rate ratio, and examining the rate ratio adjusted for dilution by considering time of diagnosis. This latter method, which is well known in the mammography screening trial literature, only includes those cancer deaths for which the corresponding time of cancer diagnosis is close enough to the end of protocol screening in the trial” (NLST Research Team, 2019). There were no stratified analyses of the dilution-adjusted lung cancer mortality rate ratio by the number of years of quitting smoking subgroups shown in the NLST study results, such as quitting smoking beyond 15 years ago. The overall results of the NLST study show that the quitting smoking history criterion for persons who had quit within the past 15 years significantly reduces lung cancer mortality among those receiving low dose CT. However, the NLST study does not have lung cancer mortality data on persons who had quit beyond 15 years ago and thus, there is no evidence from the NLST trial that supports changing or extending the quit year history to quitting more than 15 years ago.
All of the remaining low dose CT randomized controlled trials in our internal technology assessment did not include former smokers who had quit 11 to 15 years ago or who had quit beyond 15 years ago. In the good quality NELSON study, the eligibility criterion for quitting smoking history was former smokers who had quit ≤ 10 years ago (de Koning, 2020). Former smokers who had quit smoking 11 years to 15 years ago were not included and those who had quit more than 15 years ago also were not included in the NELSON study population. Among the male participants in the screening group (n = 6,583) for the NELSON study, 1.7% (49/2,908) had a quit smoking history of > 10 years since cessation of smoking (de Koning, 2020). It is not likely that the NELSON study population included men with a quit smoking history of at least 15 years or more. Additionally, the eligibility criterion for quitting smoking history did not allow former smokers who had quit smoking 11 years to 15 years ago or more than 15 years ago to be included in the NELSON study. The NELSON study does not provide data for the discussion of the quit smoking eligibility criterion for quitting smoking within the past 15 years ago.
For quitting smoking history in the ITALUNG study, “former smokers who had quit more than 10 years ago were excluded” (Paci, 2017). This exclusion criterion suggests that former smokers who had quit smoking up to 10 years ago were included and that former smokers who had quit more than 10 years ago, such as those quitting 11 to 15 years ago or quitting more than 15 years ago, were excluded from the ITALUNG study. The DANTE study (Infante, 2015) defined the eligibility criterion for quitting smoking history as persons who had quit less than 10 years before recruitment (Infante, 2015). No data were shown in the DANTE study results on the distribution of quitting smoking history for persons who had quit less than 10 years before recruitment. There were no stratified analyses of lung cancer mortality by quitting smoking history for persons who had quit less than 10 years before recruitment shown in the DANTE study results. Because the quit smoking history eligibility criterion included ex-smokers who had stopped smoking less than 10 years before the invitation to screening, the study population for the DANTE trial did not include ex-smokers who had quit within the past 11 to 15 years. Additionally, the DANTE study population did not include ex-smokers who had quit more than 15 years ago. Thus, the ITALUNG and the DANTE studies did not provide data regarding maintaining the quit smoking history eligibility criterion at quitting within the past 11 to 15 years, or quitting smoking beyond 15 years.
In the LUSI study, eligibility criterion for quitting smoking history was defined as ex-smokers who had “stopped smoking not more than 10 years before invitation to screening” (Becker, 2020). Because the quit smoking history eligibility criterion was ex-smokers who had stopped smoking not more than 10 years before invitation to screening, the study population for the LUSI trial did not include ex-smokers who had quit within the past 11 to 15 years. Additionally, the LUSI study population did
not include ex-smokers who had quit more than 15 years ago. The MILD study (Pastorino, Silva, Sestini; 2019) defined the eligibility criterion for quitting smoking history as former smoker from < 10 years ago. In the intervention arm, smoking status for smokers was defined as “former” smokers or “current” smokers with 31.4% of the study population in the intervention arm reported as “former” smokers (Pastorino, Silva, Sestini; 2019). There were no data shown on the distribution of former smokers by quit history subgroup defined as number of years quitting smoking. There were no data shown on the number of individuals who quit smoking between 10 years and 15 years ago. There were no data shown on the number of individuals who quit smoking beyond 15 years ago. For the LSS study, the eligibility criterion for quit smoking history was a former smoker who quit within the last 10 years (Doroudi, 2018). There were no data shown on the number of years since quitting smoking for the study population in the LSS study results (Doroudi, 2018). In the DLCST trial, the inclusion criterion for quitting smoking history was if participants were former smokers, they had to have quit after the age of 50 years and within the previous 10 years (Wille, 2016). Because the DLCST, LSS. LUSI, and MILD studies did not include persons who quit smoking within the past 10 or 11 to 15 years or quit smoking beyond 15 years ago, the LSS, LUSI, and MILD study results did not contribute evidence to the discussion of quit smoking history criterion.
In the UKLS study, “ex-smokers” were not defined. Among the 75,958 positive responders to the invitation letter to participate in the UKLS trial, 39.3% were ex-smokers. Among the 2,028 participants in the screening arm, 61.6% were ex-smokers (Field; May, 2016). No data on the number of years of quitting smoking was shown for the UKLS study. Because the UKLS study did not have the number of years of quitting smoking history, the UKLS study did not have data that pertains to the analysis of maintaining the quit smoking history criterion. There is no mention of a quit smoking history inclusion criterion in the methods of the secondary analysis of the COSMOS study (Rampinelli, 2017). Because there was no quit smoking history inclusion criterion and no data on lung cancer mortality, the secondary analysis of the COSMOS study did not contribute data to the evaluation of the quit smoking history eligibility criterion.
Overall, there are no lung cancer screening trial data or evidence on adults quitting smoking beyond 15 years ago. Only one study, the NLST study, shows that the quitting smoking history eligibility criterion for persons who had quit within the past 15 years significantly reduces lung cancer mortality among those receiving low dose CT. There is no relevant published human clinical study literature regarding the use of low dose CT in persons who quit smoking beyond 15 years ago. There is insufficient evidence to determine if persons who quit smoking more than 15 years ago would benefit from low dose CT screening for lung cancer. Thus, we find the human clinical evidence is sufficient to determine that screening for lung cancer with low dose CT is reasonable and necessary for the prevention or early detection of illness or disability for the population of persons who quit smoking within the past 15 years. However, we find the human clinical evidence is insufficient to determine that screening for lung cancer with low dose CT is reasonable and necessary for the prevention or early detection of illness or disability for the population of persons who quit smoking beyond 15 years ago. The study results do not show sufficient evidence to change, extend, or eliminate the eligibility criterion for the quit smoking history for lung cancer screening with low dose CT. Thus, we find that the evidence is sufficient to conclude that lung cancer screening is reasonable and necessary for Medicare beneficiaries with the specific eligibility criterion for having a history of quitting smoking within the last 15 years. The Medicare beneficiary eligibility criterion for history of quitting smoking will not be changed and will remain as quitting smoking within the last 15 years.
In the 2021 USPSTF recommendation statement, “[t]he USPSTF recommends annual screening for lung cancer with LDCT in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery. (B recommendation)” (USPSTF, Krist; 2021). The 2021 USPSTF lung cancer screening statement recommends annual lung cancer screening with low dose CT for adults who have quit within the past 15 years. This eligibility criterion for quitting smoking that is under reconsideration aligns with the 2021 USPSTF recommendation statement on low dose CT.
Among the organizations making recommendations for the selection of individuals for lung cancer screening, the American College of Chest Physicians (CHEST) has two recommendations. “1. For asymptomatic individuals age 55 to 77 who have smoked 30 pack-years or more and either continue to smoke or have quit within the past 15 years, we recommend that annual screening with low-dose CT should be offered. (Strong recommendation, moderate-quality evidence). 2. For asymptomatic individuals who do not meet the smoking and/or age criteria in Recommendation #1, are age 50 to 80, have smoked 20 pack-years or more and either continue to smoke or have quit within the past 15 years, we suggest that annual screening with low-dose CT should be offered. (Weak recommendation, moderate-quality evidence)” (Mazzone, Panel Report 2021). Thus, for the smoking cessation history for persons who have quit smoking within the past 15 years, the American College of Chest Physicians (CHEST) guidelines recommend low dose CT since this is a strong recommendation.
In the National Comprehensive Cancer Network (NCCN), Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Lung Cancer Screening Version 1.2021, the NCCN Lung Cancer Screening Panel defined the risk status of “high risk” as “age ≥ 50 years and ≥ 20 pack-year history of smoking” with these high-risk persons candidates for low dose CT (LDCT) screening (category 1) where “shared patient/physician decision-making is recommended, including a discussion of benefits/risks” (NCCN, 2020). In addition, the most current National Comprehensive Cancer Network (NCCN) guidelines Version 1.2021 do not discuss the eligibility criterion for history of quitting smoking and this eligibility criterion is not included in the most recent NCCN guidelines Version 1.2021 recommendation statement on low dose CT screening (NCCN, 2020). There were no changes to the history of quitting smoking criterion in the updated 2021 NCCN guidelines Version 1.2022 (Wood, NCCN, 2021).
In summary of their recommendations, the American College of Radiology Appropriateness Criteria expert panel concluded that for “Variant 1: Lung cancer screening with low-dose CT chest without IV contrast is usually appropriate in patients 55 to 80 years of age and 30 or more packs per year smoking history and currently smoke or have quit within the past 15 years. Variant 2: The panel did not agree on recommending lung cancer screening with low dose CT chest without IV contrast in patients 50 years of age or older and 20 or more packs per year history of smoking plus one additional risk factor. There is insufficient medical literature to conclude whether or not these patients would benefit from CT screening for lung cancer. Screening in this patient population is controversial but may be appropriate. Variant 3: Lung cancer screening is usually not appropriate in patients younger than 50 years of age or older than 80 years of age or in patients of any age with less than 20 packs per year history of smoking and no additional risk factors” (Donnelly, 2018). The American College of Radiology recommends lung cancer screening with low dose CT for persons who have quit within the past 15 years.
The American Cancer Society 2013 “guideline recommends that clinicians with access to high-volume, high-quality lung cancer screening and treatment centers should initiate a discussion about screening with apparently healthy patients aged 55 years to 74 years who have at least a 30 [or more]–pack-year smoking history and who currently smoke or have quit within the past 15 years” (Wender, 2013). However, the 2013 American Cancer Society guideline is being updated and, in the meantime, the American Cancer Society recommends yearly lung cancer screening with low dose CT scans for people who: are 50 to 80 years old and in fairly good health, currently smoke, or have quit in the past 15 years, and have at least a 20 pack-year smoking history. The American Academy of Family Physicians gives the clinical preventive services recommendation for lung cancer screening in adults a B grade recommendation. “The AAFP supports the United States Preventive Services Task Force (USPSTF) recommendation for annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 50 to 80 years who have a 20 pack-year smoking history and currently smoke or have quit within the past 15 years. Screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits life expectancy or the ability or willingness to have curative lung surgery” (AAFP, 2021). Both the American Cancer Society and the American Academy of Family Physicians recommends lung cancer screening with low dose CT for persons who have quit within the past 15 years.
We find the empiric evidence is sufficient to conclude that screening for lung cancer with low dose CT is reasonable and necessary for Medicare beneficiaries with the specific eligibility criterion for the quit smoking history of quitting smoking within the past 15 years. However, we find the human clinical evidence is not sufficient to conclude that screening for lung cancer with low dose CT is reasonable and necessary for Medicare beneficiaries with the specific eligibility criterion for the quit smoking history of quitting smoking more than 15 years ago. The Medicare beneficiary eligibility criterion for quitting smoking will not be changed and will remain at quitting smoking within the past 15 years. This recommended screening population for quitting smoking within the past 15 years does align with the 2021 USPSTF recommendation statement on low dose CT screening.
Question 3: Is the evidence sufficient to determine that screening for lung cancer with low dose computed tomography is appropriate for Medicare beneficiaries?
Lung cancer is a burdensome condition for the Medicare population due to the advanced stage and age at diagnosis and death. Early detection with various screening tests has been studied for many years without positive results but the benefits of low dose CT screening have been confirmed with extended follow up data from the high quality NLST and NELSON trials. Based on the results of the NLST and NELSON, lung cancer screening using low dose CT was shown to reduce lung cancer mortality compared to screening with chest radiogram (x-ray) or no screening, respectively, for a very defined screening population including a broader portion of the Medicare population (aged 65 or greater). The low dose CT scan is relatively simple and attempts to balance radiation exposure to acceptable image quality, compared to standard diagnostic chest CT scans that are not appropriate for lung cancer screening.
Counseling and Shared Decision-Making
Due to the complexities of lung cancer screening with LDCT including specific patient selection criteria, benefits, harms, and adherence, we continue to support the need for shared decision-making. However, we believe that the requirements should be modified to reflect a service that is no longer considered new. Professional societies and provider groups have noted that providers have gained considerable experience and expertise and believe flexibility will reduce burden. We will remove specificity regarding the type of provider who must furnish the counseling and shared decision-making and remove some specificity around documentation of the information on the beneficiary eligibility criteria.
Several organizations have commented on the type of provider who should furnish shared decision-making. The American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) policy statement (Wiener, 2015) includes comments on who should perform shared decision-making on low dose CT screening with the patient, which can include health educators or midlevel providers like lung cancer screening coordinator. The 2021 USPSTF recommendation (USPSTF, Krist; 2021) states that providers should engage in shared decision-making about screening, but there is no statement on who should conduct the shared decision-making with the patient or what level of the provider of shared decision-making should be. According to the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), they do not provide detailed comments beyond the need for a “screening program provider” on who should deliver the shared decision-making visit to the patient. While the National Comprehensive Cancer Network (NCCN) guidelines recommend shared decision-making before an initial screening LDCT scan is performed (NCCN, 2020), the guidelines do not comment on who the provider should be for the shared decision-making discussion with the patient. The updated 2021 NCCN guidelines changed the terminology for shared decision-making from “physician” to “provider” (Wood, NCCN, 2021). None of the organizations specify what type of provider should conduct shared decision-making with the patient.
We do not believe there is an evidentiary reason to continue to limit the shared decision-making visit to physician and non-physician practitioners. We note that this expansion can allow for this service to be furnished “incident to” a physician’s professional service. Removing the specification for the type of practitioner should expand the individuals that can conduct shared decision-making to other health care practitioners, such as health educators and others beyond physicians or non-physician practitioners. This change may broaden access to LDCT screening.
While we continue to have the requirement for making an appropriate determination of the patient’s eligibility for lung cancer screening with low dose CT at the shared decision-making visit, it is no longer necessary to specify the documentation requirement. We believe stating this requirement under the subheading of documentation to be redundant and that the medical record will continue to reflect a patient’s eligibility and adherence to the eligibility criteria such as calculation of cigarette
smoking pack-years and years since quitting smoking; age; and absence of signs and symptoms of lung cancer Additionally, it is routine that the patient’s age is already in the patient’s medical records, since age is a fundamental sociodemographic characteristic that should already have been entered into the patient’s medical record at the initial clinical visit. Finally, smoking history in pack-years, years since quitting smoking, and absence of signs or symptoms of lung cancer is inherent in the clinical assessment of lung cancer.
Several organizations have commented on the use of shared decision-making aids during the shared decision-making visit. According to the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), shared decision-making is suggested prior to the performance of the low dose CT screening exam, and it is suggested that ordering providers have the tools necessary to deliver an effective counseling and shared decision-making, which may include decision aids, information brochures, videos, and links to electronic resources. The American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) policy statement (Wiener, 2015) includes comments on shared decision-making as part of the low dose CT screening process and has examples of shared decision-making tools. Examples are provided of potential patient decision aid tools that could be used in shared decision-making by the provider with the patient (Wiener, 2015). While these guidelines suggest the use of shared decision aids during the shared decision-making visit, these guidelines do not specify the elements required as part of a shared decision-making tool. To reduce the burden of documentation paperwork, we will remove the specifications for the components of the shared decision-making tools. We believe this is appropriate as the tools and guidance has matured since the early inception of shared decision-making. Based on the organizations’ guidelines on shared decision-making tools, we determine that lung cancer screening with low dose CT is appropriate for Medicare beneficiaries who meet the other eligibility criteria and who participate in shared decision-making when the provider uses at least one decision aid.
We will retain the eligibility criterion on counseling on the importance of adherence to annual lung cancer low dose CT screening, the impact of comorbidities, and ability or willingness to undergo diagnosis and treatment, since, as discussed above in the introductory comments on shared decision-making, these concepts are an integral and important component of the discussion of the benefits and harms of low dose CT lung cancer screening between the patient and provider and will have a major
impact on the values attached to the patient’s preferences.
We will retain the eligibility criterion for providing information on smoking cessation interventions within the shared decision-making visit.
We are removing the excessive requirement for a written order. Additionally, having an order for the low dose CT screening test is standard practice and no longer needs to be specified as part of the NCD.
We believe that removing the restriction for the type of practitioner who can furnish the counseling and shared decision-making visit, removing the specificity of redundant documentation requirements, and eliminating the excessive requirement for a written order will reduce administrative burden and facilitate improved access to lung cancer screening with LDCT.
Smoking Cessation
We will retain the furnishing of information on smoking cessation or smoking abstinence during the counseling and shared decision-making visit, based on the guidelines published by the societal organizations. Many guideline organizations discuss smoking cessation as an important part of low dose CT screening. For annual lung cancer screening with low dose CT in adults, the 2021 USPSTF recommendation has that if a person currently smokes, they should receive smoking cessation interventions. Further, “persons referred for lung cancer screening through primary care should receive these interventions concurrent with a referral. Because many persons may enter screening through pathways besides referral from primary care, the USPSTF encourages incorporating such interventions into all screening programs” (USPSTF, Krist; 2021). However, the 2021 USPSTF recommendation does not state within what setting that smoking cessation should occur in except that smoking cessation should occur within the setting of the screening program. The 2021 USPSTF recommendation also discusses other resources on smoking cessation developed by the Centers for Disease Control and Prevention and the National Cancer Institute.
In the section on shared decision-making, the National Comprehensive Cancer Network (NCCN) guidelines state that “smoking cessation counseling is recommended” (NCCN, 2020). Thus, while the NCCN guidelines recommend advising smokers to quit smoking tobacco, the guidelines do not explicitly comment on whether the discussion of smoking cessation should be part of shared decision-making. Additionally, the NCCN guidelines do not comment on the setting for the discussion of smoking cessation or when and where to discuss quitting smoking with the patient. The issue of setting remains unchanged in the updated 2021 NCCN guidelines (Wood, NCCN, 2021). According to the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), evidence-based tobacco cessation treatment is recommended to be provided by the low dose CT screening program, even though the panel reported that the most effective intervention to promote smoking cessation in the setting of lung cancer screening is not known. The American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) policy statements include comments on smoking cessation, but the statement notes that “the best strategy to optimize smoking abstinence in the setting of LDCT screening is unknown” (Wiener 2015). The ATS/CHEST policy statement (Wiener, 2015) does not comment on the setting, such as the specific imaging facility, for discussing smoking cessation with the patient or whether it should be included in shared decision-making. However, one organization suggests that smoking cessation intervention be integrated with shared decision-making. In the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), counseling about smoking cessation is suggested as a component of the
counseling and shared decision-making visit of the patient with the clinical provider.
We will retain the requirement for furnishing information about tobacco cessation interventions or maintaining cigarette smoking abstinence within the shared decision-making visit. Given the current rates of smoking, we believe it is vitally important to provide information about tobacco cessation or cigarette smoking abstinence to the patient when possible. Based on the organizations’ guidelines on smoking cessation for low dose CT screening, we will retain the eligibility criterion that lung cancer screening with low dose CT is appropriate for Medicare beneficiaries who meet the other eligibility criteria and who have furnished information about tobacco cessation interventions if current smoker, or maintaining cigarette smoking abstinence if former smoker, during the shared decision-making visit. We determine that as a policy requirement for low dose CT screening as a radiology imaging facility eligibility criterion, it is not required for Medicare beneficiaries to receive smoking cessation interventions for current smokers within the setting of a radiology imaging facility, and that this eligibility criterion be removed from the radiology imaging facility eligibility criteria as part of this NCD.
Written Order
We will modify the criterion for a written order by removing the word “written” and removing the data elements that are required as part of the order. As stated above, orders for lung cancer screening with LDCT are now standard practice and we no longer believe it is necessary to specify each data element on the order.
While it is important and necessary for the facility to receive an order for the appropriate Medicare beneficiary to have a low dose CT scan to screen for lung cancer, the order does not have to be written since it is more likely that an order is transmitted electronically with the use of an electronic health record system. Additionally, the information we had previously specified is available in the medical record such as smoking history and years since quitting smoking, is in the patient’s medical records. Another reason for removing the detailed data elements is that we are removing the low dose CT lung cancer screening registry requirement and many of these elements were required as part of the registry data submission.
Written Orders for Subsequent Annual Lung Cancer Screenings with Low Dose CT
We will remove the requirement for written orders for subsequent annual lung cancer screening with low dose CT. Consistent with our previous discussion, we believe that specifying the order be “written” is outdated and that orders for subsequent annual lung cancer screening with LDCT are standard practice and are no longer necessary as part of the NCD.
Reading Radiologist Training and Eligibility Criteria
We will modify the reading radiologist eligibility criteria by removing the training documentation requirement, the 300 chest CT acquisitions in 3 years requirement, the documented participation in continuing medical education in accordance with current American College of Radiology standards requirement, and the radiology facility eligibility criteria.
We will retain the eligibility criterion that the reading radiologist must be board certified or board eligibility with the American Board of Radiology or equivalent organization in order to maintain the standards of the American Board of Radiology, the national medical specialty board that certifies radiologists. The mission of the American Board of Radiology is to “certify that our diplomats demonstrate the requisite knowledge, skill, and understanding of their disciplines to the benefit of
patients. . . . We were founded to protect the public by assessing and certifying doctors who meet specific educational, training, and professional requirements” (American Board of Radiology, 2021). Further, “[b]oard certification is the best measure of the knowledge, experience, and skills needed to provide quality patient care” (American Board of Radiology, 2021). To maintain the standards of the American Board of Radiology, we will retain the eligibility criterion that lung cancer screening with low dose CT is appropriate for Medicare beneficiaries who meet the other eligibility criteria when the reading radiologist has board certification or board eligibility with the American Board of Radiology or equivalent organization.
For initial board certification in diagnostic radiology, the requirements are that “[a]s candidates progress through their residency and after completion of training, they will take two exams to gain initial certification in diagnostic radiology: the Core Exam and the Certifying Exam” (American Board of Radiology, 2021). There is no mention of a requirement for additional training beyond residency training in diagnostic radiology and radiation safety, or the need for interpretation of at least 300 chest computed tomography acquisitions in the past three years. There is no subspecialty in CT scans. Thus, we will remove the eligibility criterion that the reading radiologist has documented training in diagnostic radiology and radiation safety, and to remove the other eligibility criterion that the reading radiologist be involved in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years, which should reduce the documentation paperwork burden on the provider.
Further, we will remove the eligibility criterion that the reading radiologist has documented training in diagnostic radiology and radiation safety because this requirement is part of the requirements for maintaining board certification in diagnostic radiology. Diagnostic radiology is one of the medical specialties that a radiologist can attain board certification from the American Board of Radiology. To maintain board certification in diagnostic radiology, the radiologist is required to complete training for the Maintenance of Certification for Diagnostic Radiology. “Maintenance of Certification (MOC) is an integral part of the quality movement in healthcare. Patients, your physician peers, and your colleagues all value MOC because it demonstrates your support for continuous quality improvement, professional development, and quality patient care. The ABR [American Board of Radiology] believes in the value of Maintenance of Certification. Therefore, all ABR volunteers, including governors and trustees, are required to participate in MOC” (American Board of Radiology, 2021). Maintenance of Certification includes documented training requirements for lifelong learning, and a self-assessment of knowledge, judgment and skills in diagnostic radiology, which includes radiation safety. Because maintenance of board certification in diagnostic radiology incorporates documented training in diagnostic radiology and radiation safety, we will remove the eligibility criterion that the reading radiologist has documented training in diagnostic radiology and radiation safety, which should reduce the documentation paperwork burden on the provider.
Additionally, none of the guideline organizations mention a need for the reading radiologist for CT scans to be involved in a specific number of chest computed tomography readings. We will remove the eligibility criterion that the reading radiologist be involved in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years, which should reduce unnecessary requirements for the provider.
After reviewing public comments, we will remove the eligibility criterion that the reading radiologist must have documented participation in CME in accordance with current American College of Radiology standards. CME is already part of the American Board of Radiology’s Maintenance of Certification (MOC) Part 2 that requires radiologists seeking board re-certification to attain 75 CME credits every three years. Since the reading radiologist is already required, per the NCD, to be board certified or board eligible, we will not continue a separate CME requirement for reading radiologists.
We will remove the reading radiologist eligibility criterion for furnishing lung cancer screening with low dose CT in a radiology imaging facility that meets the radiology imaging facility eligibility criteria because we are removing all but one of the radiology imaging facility eligibility criteria, which should reduce the burden of work on the provider. The rationale for removing radiology imaging facility eligibility criteria is described in the section on the low dose CT lung cancer screening imaging facilities that follows.
Low Dose CT Lung Cancer Screening Imaging Facilities, Low Dose CT Lung Cancer Screening Registry, and Radiation Dose
We will modify the radiology imaging facility eligibility criteria. Lung cancer screening with LDCT is now a mature technology that no longer requires the criteria established early in its inception.
Low Dose CT Lung Cancer Screening Imaging Facilities: Radiation Dose
We will remove the radiology imaging facility eligibility criteria for volumetric CT dose index by the number of milligrays, based on recently published guidelines. Several multi-society multi-disciplinary guideline organizations, including major professional medical societies such as the American College of Radiology, have comments on radiation dosage. The American College of Radiology (ACR) Appropriateness Criteria expert panel appears to make a statement related to the radiology imaging screening facility low dose CT eligibility criteria that suggests what the specific number of milliSieverts for radiation dosing should be (Donnelly, 2018), likely applicable to independent diagnostic testing facilities (IDTFs). The American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021) has suggestions about radiology imaging screening facility low dose CT eligibility criteria, such as the number of milligrays for radiation dosing. According to the American College of Chest Physicians (CHEST) guidelines for LDCT lung cancer screening, it is suggested “that low-dose CT screening programs follow the ACR/STR (American College of Radiology and Society of Thoracic Radiology) protocols for performing low radiation dose chest CT scans. (Ungraded Consensus-Based Statement). Remark: An awareness of the potential for radiation related harm can help programs thoughtfully plan ways to minimize this risk through proper patient selection, the performance of the CT scan, tracking of the radiation dose being administered, and appropriate management of screen detected findings” (Mazzone, Panel Report 2021).
Additionally, the American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) policy statements (Wiener, 2015) include comments on radiology imaging screening facility low dose CT eligibility criteria, such as the number of milligrays for radiation dosing (Wiener, 2015), likely for independent diagnostic testing facilities (IDTFs). Radiology imaging screening facility eligibility criteria, such as radiation dosing by low dose CT, is discussed in the body of the current National Comprehensive Cancer Network (NCCN) guidelines, but it is noted as a footnote in the clinical pathway algorithm for lung cancer screening, and is not part of the topline recommendation statement as part of the eligibility criteria for low dose CT lung cancer screening, of which age and pack-year history of smoking are such topline criteria. Further, the current NCCN guidelines state that “to help ensure good image quality, all chest LDCT screening program should use CT scanners that meet the standards of the American College of Radiology (ACR)” (NCCN, 2020). There were no changes to radiation dose in the updated 2021 NCCN guidelines (Wood, NCCN, 2021).
Multi-society multi-disciplinary organizations with extensive expertise in low dose CT scans, such as the American College of Radiology, have published guidelines on the radiation dose that should be emitted by the low dose CT scan. Additionally, the guidelines appear to support the effort to standardize the protocol for administering the low dose CT scan, including the radiation dosing, indicating a maturing technology. Thus, we will remove the radiology imaging facility eligibility criteria for volumetric CT dose index by the number of milligrays, which is likely to reduce the burden of documentation paperwork on providers and institutions.
Low Dose CT Lung Cancer Screening Imaging Facilities: Lung Nodule Reporting System
We will not finalize the proposal to remove the radiology imaging facility eligibility criteria for utilizing a standardized lung nodule identification, classification and reporting system, based on guidelines published by multi-society multi-disciplinary stakeholders. Several multi-society guideline organizations have comments on utilizing a standardized lung nodule identification, classification and reporting system that focuses primarily on the reporting system developed by the American College of Radiology. The 2021 USPSTF recommendation reports that to standardize low dose CT screening and the evaluation and management of abnormal lung nodule findings, the USPSTF endorses the use of the American College of Radiology Lung Imaging Reporting and Data System (Lung-RADS) classification system for lung cancer screening with low dose CT in adults (USPSTF, Krist; 2021). According to the recent American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), there is a suggestion to use a lung nodule reporting and management system such as the American College of Radiology Lung-RADS, likely useful for independent diagnostic testing facilities (IDTFs). Similarly, the American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) policy statement (Wiener, 2015) include comments on radiology imaging screening facility low dose CT eligibility criteria, such as the use of the Lung-RADS lung nodule reporting and management system (Wiener, 2015), Radiology imaging screening facility eligibility criteria, such as the use of Lung-RADS in lung nodule reporting and management, is discussed in the body of the current National Comprehensive Cancer Network (NCCN) guidelines, but it is noted as a footnote in the clinical pathway algorithm for lung cancer screening, and not part of the topline recommendation statement as part of the eligibility criteria for low dose CT lung cancer screening, of which age and pack-year history of smoking are such topline criteria. The updated 2021 NCCN guidelines contain detailed recommendations for evaluating and follow up on lung screening findings including immediate assessment of nodules that are highly suspicious for lung cancer. The 2021 NCCN guidelines and the Lung-RADS recommendations have been harmonized to provide consistent and clear recommendations to clinicians seeking to interpret low dose CT scans (Wood, NCCN, 2021).
Societal organizations with expertise in low dose CT scans have published guidelines recommending the use of a lung nodule reporting and management system, such as the American College of Radiology Lung Imaging Reporting and Data System (Lung-RADS) classification system. Given that societal organizations with extensive expertise in low dose CT scans have opined about the use of a lung nodule reporting and management system, and more specifically in response to the public comments, the radiology imaging facility eligibility criterion for utilizing a standardized lung nodule identification, classification and reporting system will remain. This is likely to standardize LDCT screening and the evaluation and management of abnormal lung nodule findings, and as noted in the public comments, reduce ambiguity in follow up and clinical management recommendations made to ordering providers by providing clear and consistent communication between the reading radiologist and the ordering providers.
Low Dose CT Lung Cancer Screening Imaging Facilities: Smoking Cessation
As noted in the above section on smoking cessation, we determine that as a policy requirement for low dose CT screening as a radiology imaging facility eligibility criterion, it is not required for Medicare beneficiaries to receive smoking cessation interventions for current smokers within the setting of a radiology imaging facility, and that this eligibility criterion be removed from the radiology imaging facility eligibility criteria as part of this NCD. This modification should simplify and streamline the patient workflow for lung cancer screening at the radiology imaging facility.
Low Dose CT Lung Cancer Screening Registry
We will remove the radiology imaging facility eligibility criteria for collecting and submitting data to a CMS-approved low dose CT lung cancer screening registry along with the minimum required data elements. From the 2015 decision memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT), “[t]he primary purpose for requiring the submission of data to the registry is to document compliance with the coverage criteria that are not evidenced on the health care claim. Furthermore, based on the public comments and the evidence reviewed, we strongly believe that the registry will serve as an aid to those seeking to study the clinical benefits of this screening” (CAG-00439N; CMS,
2015).
For the purpose of showing the benefit of low dose CT screening, three studies were published using the Lung Cancer Screening Registry administered by the American College of Radiology. Pham et al. (2020) used “data from the Lung Cancer Screening Registry provided by the American College of Radiology in 2016” to collect “the total number of LDCT screens performed from all 1962 accredited radiographic screening sites. The 2015 National Health Interview Survey was used to estimate screening eligible smokers per United States Preventive Services Task Force criteria. The number of LDCT screens performed in the US was collected from the 2016 Lung Cancer Screening Registry (LCSR), a part of The American College of Radiology (ACR). Started in 2015, this registry is based on the ACR Lung Imaging Reporting and Data System, which is a quality assurance tool designed to standardize the reporting of LDCT lung cancer screenings, manage recommendations, reduce confusion in LDCT interpretations, and facilitate outcome monitoring. LCSR is approved by CMS to enable providers to meet quality-reporting requirements for Medicare LDCT lung cancer screening reimbursement” (Pham, 2020). They found that “[i]n 2016, 2.0% of 7.6 million eligible smokers were screened” with low dose CT across the United States (Pham, 2020). The authors concluded that “[l]ung cancer screening remains heavily underutilized despite [the USPSTF] guideline recommendation since 2013, insurance coverage, and its potential to prevent thousands of lung cancer deaths annually” (Pham, 2020). Additionally, Gupta et al. (2021) conducted a quasi-experimental study to compare the annual growth rates in lung cancer screenings between states that expanded Medicaid versus those that did not. “Using the American College of Radiology Lung Cancer Screening Registry, they calculated the average annual growth rate between 2016 and 2019 for both groups” (Gupta, 2021). The American College of Radiology Lung Cancer Screening Registry “is a nationwide registry of accredited screening sites that perform lung cancer screenings. It was created in 2015, is publicly available and has been used to generate national estimates of lung screening coverage rates” (Gupta, 2021). The authors found that “[n]o significant difference was identified in the average annual growth in lung cancer screenings between Medicaid expanding and non-expanding states (57.6%, 50.3%, p = 0.51)” (Gupta, 2021). The authors concluded that “[n]o association is found between Medicaid expansion and increasing volumes of lung cancer screenings between 2016 and 2019” (Gupta, 2021). Finally, the aim of the Charkhchi (2017) study was “to assess the relationship between the availability of lung cancer screening facilities and the size of the at-risk population as well as state-level clinically relevant epidemiologic and demographic variables”. “The ACR LCSR [American College of Radiology Lung Cancer Screening Registry] list of participating lung cancer screening facilities was used as a proxy for the availability of lung cancer screening facilities in each state” (Charkhchi, 2017). The authors reported that “[c]urrently, the only CMS-approved lung cancer screening registry is the Lung Cancer Screening Registry (LCSR) administered by the American College of Radiology (ACR). LCSR registers practices that provide LDCT and collects standardized information regarding those individuals who receive lung cancer screening in these registered practices” (Charkhchi, 2017). The authors found that “[a]s of 11/18/2016, 2,423 facilities participated in the LCSR. When adjusted by the rate of screening-eligible individuals per 100,000, the median population-normalized facility number was 15.7 (interquartile range (IQR) 25%, 75% 10.7,19.3). There was a positive independent effect (coefficient=12.87, 95% CI= 10.93–14.8) between the state-level number of screening facility and rate of screen-eligible individuals rate per 100,000” (Charkhchi, 2017). The authors concluded that “[f]acility number [at the state level] correlated with the rate of screening-eligible individuals per 100,000, a measure of the at-risk population” (Charkhchi, 2017).
Thus, three studies used the American College of Radiology Lung Cancer Screening Registry to ascertain how the rates of low dose CT utilization were affected by Medicaid expansion (Gupta, 2021), the association between the geographic distribution of lung cancer screening facilities and the rate of screening eligible individuals (Charkhchi, 2017), and most importantly, that lung cancer screening remains heavily underutilized despite the positive USPSTF guideline recommendation since 2013 (Pham, 2020). We believe the results of the three studies fulfill the purpose of the low dose CT lung cancer screening registry.
Several societal organizations provided comments on whether there is a need for a low dose CT lung cancer screening registry. For annual lung cancer screening with low dose CT in adults, the more recent 2021 USPSTF recommendation has no comments on the use of a low dose CT lung cancer screening registry (USPSTF, Krist; 2021). In the previous 2013 USPSTF recommendation under Other Considerations for Standardizing LDCT Screening and Follow-up of Abnormal Findings, “in the context of substantial uncertainty about how best to manage individual lesions, as well as the magnitude of some of the harms of screening, the USPSTF encourages the development of a registry to ensure that appropriate data are collected from screening programs to foster continuous improvement over time” (Moyer, USPSTF; 2014). However, these sentences on the development of a registry do not appear in the 2021 USPSTF recommendation under “Standardizing LDCT Screening and Follow-up of Abnormal Findings” (USPSTF, Krist; 2021).
In the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening (Mazzone, Panel Report 2021), they make suggestions to develop data collection and reporting tools capable of assisting with quality improvement initiatives and reporting to a national registry, but reporting to a registry is likely linked to the 2015 CMS requirement for a national registry, as mentioned in the evidence base for this CHEST guideline. Further, this comment was an “ungraded consensus-based statement, and not a recommendation. The American Thoracic Society (ATS) and American College of Chest Physicians (CHEST) policy statement concludes that “to maintain performance, programs should collect data on patients undergoing LDCT screening in a registry that should be periodically reviewed to ensure the program is achieving quality metrics” (Wiener, 2015). The ATS/CHEST policy statement reports that “regardless of nodule size, it is essential to establish mechanisms to prevent loss to follow-up. Two such strategies are the development of registries with data on all patients who have undergone LDCT screening (a CMS requirement) and designation of a screening program coordinator. Registries help monitor recommended follow-up, whether for a repeat screening in 1 year or earlier testing for evaluation of a screen-detected nodule” (Wiener, 2015). But similar to the CHEST guidelines, the registry requirement appears to be included in the ATS/CHEST policy statement (Wiener, 2015) because the requirement is included in the 2015 national coverage determination on lung cancer screening as a screening facility eligibility criterion.
However, two guideline organizations do not comment on a low dose CT lung cancer screening registry. The current National Comprehensive Cancer Network (NCCN) guidelines Version 1. 2021 (NCCN, 2020) do not comment on the necessity of registries for low dose CT lung cancer screening. No changes regarding registries are noted in the updated 2021 NCCN guidelines (Wood, NCCN, 2021). Additionally, the American College of Radiology (ACR) Appropriateness Criteria expert panel (Donnelly, 2018) does not make any statements about registries for low dose CT lung cancer screening.
Thus, we will remove the criteria for imaging facilities to participate in a CMS-approved low dose CT lung cancer screening registry.
Given that three published studies use the Lung Cancer Screening Registry administered by the American College of Radiology fulfilled the purpose as outlined in the previous NCD and that the most recent 2021 USPSTF recommendation statement has changed to having no comment on the need for a lung cancer registry, we will remove the radiology imaging facility eligibility criteria for collecting and submitting data to a CMS-approved low dose CT lung cancer screening registry which is likely to reduce the burden of administrative paperwork on providers and institutions.
Health Disparities
Lung cancer incidences are more common in men than women and highest in African American men (NCI, 2021).
(NCI, 2021)
Among the randomized controlled trials in our evidence base, one study, the NLST, had data on African-Americans, who comprised 4.4% of the study population (NLST, 2019). Women comprised 16.4% (NELSON; de Koning, 2020) to 44.8% (DLCST; Wille, 2016) of the study populations.
Regarding the issues underlying health disparities in lung cancer screening, Krist and colleagues (2021), on behalf of the USPSTF, reported that “African American/Black (Black) men have a higher incidence of lung cancer than White men, and Black women have a lower incidence than White women. These differences are likely related to differences in smoking exposure (i.e., prevalence of smoking) and related exposure to carcinogens in cigarettes. The differences may also be related to other social risk factors” (USPSTF, Krist; 2021).
Additionally, in the American College of Chest Physicians (CHEST) guidelines for low dose CT lung cancer screening, the authors reported that “[a]mong patients enrolled in the NLST, individuals who currently smoke and black subjects experienced the highest lung cancer mortality and the greatest benefit from LDCT screening. However, minorities and those with low SES [socioeconomic status] (who are more likely to be currently smoking) often experience disparities in receiving appropriate preventive health care. LDCT screening has been slow to be implemented and is underused nationally despite coverage by private and public insurers. Lower rates of screening uptake have been found among minorities, those with a lower educational status, and individuals with low SES. As screening is implemented more widely, outreach to underserved populations to ensure that eligible individuals receive LDCT screening will be of critical importance to prevent disparities. Little work has been done to establish the most effective strategies” (Mazzone, Executive Summary; 2021).
More specifically on smoking and race and gender disparities, the authors of the American Thoracic Society (ATS) statement reported that “incidence and mortality rates vary by race, ethnicity, and sex. African American and Native Hawaiian individuals have the highest incidence, and white individuals have midlevel incidence, whereas Hispanic and Asian individuals have the lowest rates. These differences in incidence are more evident by sex at low levels of smoking exposure and at younger ages. Although the national decline in the incidence of lung cancer among men has been greater than that among women, in the 40- to 44-year-old age group, the female-to-male incidence rate ratio of lung cancer increased from 0.82 in the 1995–1999 period to 1.13 in the 2010–2014 period. The crossover in rates to higher incidence rates in younger women occurred in birth cohorts born after 1965 and was limited to white and Hispanic individuals; among white individuals, the rate rose from 0.88 in the 1995–1999 period to 1.17 in the 2010–2014 period, and among Hispanic women, who smoke less than young Hispanic men, the rate rose, more notably, from 0.79 to 1.22. African American men have the highest lung cancer mortality of all groups, and lung cancer is the leading cause of mortality in Hispanic men and the second leading cause of cancer mortality in Hispanic women. Socioeconomic and racial disparities account for approximately 37% of premature cancer deaths in the United States and are a major public health concern. The largest socioeconomic disparity is reported for lung cancer with mortality rates five times higher in the least educated men than in the most educated men. Secondary analyses of the NLST data show that African American individuals are more likely to benefit from LCS [lung cancer screening] in terms of mortality reduction (hazard ratio, 0.61 in African American individuals vs. 0.86 in white individuals). In addition, the reduction in lung cancer mortality after LCS is suggestive of being more favorable in women than in men” (Rivera, 2020).
For health disparities by gender in lung cancer screening, as noted earlier in the NCA, Becker et al. (2020) reported “an intriguing observation in LUSI [in that there] is the apparent heterogeneity (although only borderline significant, P value for heterogeneity= 0.09) in the effect of LDCT screening on lung cancer mortality by sex, suggesting a mortality reduction among the women only,” (Becker, 2020) and the authors go on to report that the “findings from LUSI are in line with those from other trials, including NLST [National Lung Screening Trial], that suggest a stronger reduction of lung cancer mortality after LDCT screening among women as compared to men” (Becker, 2020). Additionally, the LUSI study authors report that this gender “heterogeneity could be the result of different relative counts of lung tumor [histologic] subtypes occurring in men and women,” but that the “numbers of cancer deaths in LUSI were too small to examine whether the apparent heterogeneity in relative mortality hazards for men and women could be explained by differences in tumor histology, or whether it could have been entirely due to chance” (Becker, 2020).
Regarding the impact of health disparities on eligibility for lung cancer screening, the authors of the American Thoracic Society (ATS) statement reported that “[r]acial and ethnic differences in lung cancer risk that are not accounted for in current eligibility criteria for LCS are striking. African American individuals exhibit higher smoking-adjusted risk of cancer, despite smoking less than white individuals. Recent data from a large cohort study demonstrated that lung cancer cases in African American individuals were less likely to be eligible under USPSTF screening guidelines than lung cancer cases in white individuals (17% vs. 31%, respectively), primarily because of fewer pack-years smoked” (Rivera, 2020). Further, “[s]creening eligibility also does not account for sex-based differences in lung cancer risk. Lung cancer tends to be diagnosed in women at younger ages than in men, and women start smoking at a later age and smoke less intensively than men. A recent study reported that although smoking prevalence was lower in women born after 1965, the incidence rate of lung cancer was significantly higher than in men, especially in white and Hispanic women, suggesting that sex differences in smoking behavior do not fully explain increased lung cancer rates in young women” (Rivera, 2020).
Strategies to Address Health Disparities
To address health disparities in lung cancer screening, the authors of the American Thoracic Society (ATS) statement on healthcare disparities reported that “[d]isparities in lung cancer incidence, diagnosis, treatment, and mortality are well documented. There is concern that disparities in the implementation of and access to lung cancer screening (LCS) will further widen existing gaps in lung cancer care and mortality among racial and ethnic minorities, individuals of low socioeconomic status (SES), and uninsured or underinsured populations” (Rivera, 2020). One of the key conclusions of the ATS statement was that “in the United States, lung cancer incidence and mortality rates vary by race, ethnicity, sex, and SES [socioeconomic status]. LCS saves lives, and the mortality reduction benefit has been shown to be more favorable in African American individuals than in white individuals and is suggestive of being more favorable in women than in men” (Rivera, 2020).
Additionally, the authors of the American Thoracic Society (ATS) statement on healthcare disparities suggest several strategies to address health disparities in lung cancer screening. “Healthcare providers and organizations should provide access to evidence-based tobacco treatment that includes behavioral counseling and should develop programs that address differences in cultural beliefs, language, and literacy; healthcare institutions should provide training for providers on communication techniques in LCS [lung cancer screening] SDM [shared decision-making] to build and improve patient trust[; and] research scientists and healthcare providers should develop and test SDM tools that are culturally sensitive and understandable by those with lower literacy and numeracy and by those of differing cultural backgrounds” (Rivera, 2020). The American Thoracic Society statement concludes that “[s]ocially and economically disadvantaged populations are among the most vulnerable populations at risk for poor lung cancer outcomes. Significant disparities across the continuum of LCS implementation—not getting screened for tobacco use, not meeting eligibility criteria, not having access to quality screening and tobacco treatment, and lack of insurance, among many—threaten to worsen disparities in lung cancer. Dedicated efforts are needed to address existing multilevel barriers to LCS that widen disparities and to respond and develop actionable plans to implement strategies using multipronged approaches deployed simultaneously to decrease disparities. Thoughtful implementation of strategies that address racial, ethnic, socioeconomic, and sex-based differences in smoking behaviors and lung cancer risk; address inequitable distribution of LCS resources and access to health insurance coverage for LCS; and provide education and resources will be necessary to achieve equitable outcomes in LCS” (Rivera, 2020).
CMS encourages shared decision-making between patients and practitioners that is culturally sensitive and understandable by those with lower literacy and numeracy and by those of differing cultural backgrounds; and if appropriate, participation in a lung cancer screening program. CMS also encourages the use of tobacco cessation and abstinence programs that address differences in cultural beliefs, language, and literacy.
Modification of Eligibility Criteria for Age and Smoking History
Two studies evaluated the impact of the revised USPSTF eligibility criteria for age and smoking history on health disparities in lung cancer screening. In an ongoing prospective observational cohort study of community health centers across 12 southern US states, there were a total of 48,364 Southern Community Cohort Study participants who were ever smokers, of which “5,654 of 32,463 African American smokers (17%) were eligible for USPSTF screening compared with 4,992 of 15,901 white smokers (31%) (P < .001)” (Aldrich, 2019). The study authors found that “[l]owering the smoking pack-year eligibility criteria to a minimum 20-pack-year history was associated with an increased percentage of screening eligibility of African American smokers” from 17.4% to 28.5% (Aldrich, 2019). This finding was likely due to “African American smokers [tending] to smoke fewer cigarettes per day compared with white smokers and [having] a lower overall smoking pack-year history” (Aldrich, 2019). Additionally, “[r]educing the minimum age criterion for screening to 50 years for African American smokers in addition to lowering the smoking pack-year requirement would further increase the eligibility of African American persons diagnosed with lung cancer to 57.8% (457 of 791)” (Aldrich, 2019). The authors concluded that “[c]urrent USPSTF lung cancer screening guidelines may be too conservative for African American smokers. The findings suggest that race-specific adjustment of pack-year criteria in lung cancer screening guidelines would result in more equitable screening for African American smokers at high risk for lung cancer” (Aldrich, 2019).
Reese et al. (2021) conducted a study to determine “[w]hat change will be associated with the revised US Preventive Services Task Force (USPSTF) lung cancer screening guideline for screening eligibility among female, Black, and Hispanic populations” (Reese, 2021). “In the revised criteria, age was modified to 50 to 80 years [from 55 to 80 years]; smoking history, to 20 pack-years” (Reese, 2021). The authors found that “[i]n this cross-sectional study of the [Centers for Disease Control and Prevention’s] Behavioral Risk Factor Surveillance System for 2017 and 2018 [across 19 states], the proportion eligible for screening among current and former smokers increased by 30.3% for men, 40.5% for women, and 31.9% for White, 76.7% for Black, and 78.1% for Hispanic populations. Compared with men, women had lower odds of eligibility, and compared with White [individuals], Black and Hispanic individuals had lower odds of eligibility” (Reese, 2021). The authors concluded that “[t]he revised USPSTF guideline may likely increase lung cancer screening rates for female, Black, and Hispanic populations. However, despite these potential improvements, lung cancer screening inequities may persist without tailored eligibility criteria” (Reese, 2021).
Several societies and the USPSTF have developed statements on the impact of changing the lung cancer screening eligibility criteria for age and smoking history. In the American College of Chest Physicians CHEST) guidelines for low dose CT lung cancer screening, the authors reported that “[l]imited data comparing lung cancer mortality outcomes by race, smoking status, malignancy risk, and the presence of COPD were available. The NLST was the only trial for which there is data reporting lung cancer mortality stratified by race. Black individuals had a non-statistically significant larger benefit [from lung cancer screening] than white individuals (HR 0.61 vs. 0.86, p = 0.29)” (Mazzone, Panel Report 2021). Additionally, “[c]urrent age and smoking history-based eligibility criteria engender disparities with respect to race/ethnicity, sex, smoking intensity, years since quitting, and for special populations such as people living with HIV; see [citation in Mazzone] for a comprehensive review. By reducing the age and the pack-years eligibility for screening from 55 to 50 and 30 to 20, respectively, as in the USPSTF draft recommendations, more African Americans will be eligible for screening which may partially eliminate this particular disparity” (Mazzone, Executive Summary; 2021).
In agreement with the American College of Chest Physicians (CHEST) guidelines, the American Thoracic Society (ATS) statement on healthcare disparities reported that “[l]ung cancer is diagnosed in African American individuals, women, and PLHIV [persons living with HIV] at an earlier age than white individuals, men, and non-HIV populations, and the former populations may thus benefit from a lower minimum age for eligibility. Decreasing the eligibility criteria for smoking pack-years to a minimum of 20 pack-years has been shown to increase the percentage of African Americans who smoke who would be eligible for LCS. Further reducing the eligible age to 50 years for African Americans in addition to allowing a minimum history of 20 pack-years resulted in a similar eligibility percentage between African American individuals and white individuals in whom lung cancer was diagnosed. Current USPSTF LCS guidelines under select women, who are more likely to smoke with low intensity or be former high-intensity smokers with >15 YSQ [Years Since Quitting]. Modifying the smoking history and/or age eligibility criteria for LCS, which the 2020 [sic] USPSTF draft recommendations propose, would increase the percentage of lung cancer cases eligible for screening; however, there is not sufficient evidence that this proposal alone will ensure equitable screening for all individuals who have equal risk of lung cancer” (Rivera, 2020).
Finally, regarding the impact of changing the eligibility criteria for age and smoking history, Krist and colleagues (2021), on behalf of the USPSTF, concluded in a similar fashion to the above societal guideline statements that “[s]creening for lung cancer in persons at an earlier age and with fewer pack-years of smoking (i.e., 20 pack-years) may also help partially ameliorate racial disparities in screening eligibility. Data suggest that Black persons who smoke have a higher risk of lung cancer than do White persons, and this risk difference is more apparent at lower levels of smoking intensity. One recent analysis of Southern Community Cohort Study participants found that 17% of Black persons who smoke were eligible for lung cancer screening based on the 2013 USPSTF eligibility criteria compared with 31% of White persons who smoke. In the same study, among persons diagnosed with lung cancer, a significantly lower percentage of Black persons who smoke (32%) were eligible for screening than were White persons (56%). Data also suggest that Latinx/Hispanic persons who smoke accumulate fewer pack-years than White persons who smoke. A strategy of annually screening persons aged 50 to 80 years who have at least a 20 pack-years smoking history and currently smoke or have quit within the past 15 years (A-50-80-20-15) would increase the relative percentage of persons eligible for screening by 87% overall—78% in non-Hispanic White adults, 107% in non-Hispanic Black adults, and 112% in Hispanic adults compared with 2013 USPSTF criteria (A-55-80-30-15). Similarly, a strategy of screening persons aged 50 to 80 years who have at least a 20 pack-year smoking history and currently smoke or have quit within the past 15 years (A-50-80-20-15) would increase the relative percentage of persons eligible for screening by 80% in men and by 96% in women, because they accumulate fewer pack-years than men.” (USPSTF, Krist; 2021).
The available evidence, including the health disparity study results and the societal organization’s clinical guideline statements, suggest that the CMS proposal to revise the lung cancer screening eligibility criteria by lowering the starting age to 50 years and reducing the smoking history to 20 pack-years may help to partially ameliorate gender and race/ethnicity-related health disparities in eligibility for lung cancer screening.
Summary
Given the burden of lung cancer on the United States population, a suitable screening test for lung cancer has been sought for many years. Lung cancer screening has been recommended by the USPSTF with a grade B recommendation for certain individuals. Based on our review of the available evidence, including clinical guidelines and public comments, we find that the evidence is sufficient to conclude that broadening the eligibility criteria for lung cancer screening with low dose CT is reasonable and necessary for the prevention or early detection of illness or disability and appropriate for Medicare beneficiaries under conditions established in this NCD. These conditions are supported by the evidence reviewed, including conditions in the randomized controlled clinical trials and evidence-based multi-society, multi-disciplinary recommendations. The results of ongoing trials will provide additional evidence. We believe that specific beneficiary and practitioner eligibility requirements are necessary to ensure that the benefits of screening outweigh harms in the Medicare population, consistent with the various randomized controlled clinical trials. Lung cancer screening with low dose CT has not been implemented broadly in any population to date. While we are establishing coverage for this additional preventive service under Medicare part B, we believe we need to proceed in a responsible manner and will continue to monitor the evidence as stewards of the Medicare program.
X. Conclusion
The Centers for Medicare & Medicaid Services (CMS) reconsidered the national coverage determination established at section 210.14 of the Medicare National Coverage Determinations manual and determines that the evidence is sufficient to expand the eligibility criteria for Medicare beneficiaries receiving low dose computed tomography (LDCT) when the following criteria are met:
Beneficiary eligibility criteria:
- Age 50 – 77 years;
- Asymptomatic (no signs or symptoms of lung cancer);
- Tobacco smoking history of at least 20 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
- Current smoker or one who has quit smoking within the last 15 years; and
- Receive an order for lung cancer screening with LDCT.
Counseling and Shared Decision-Making Visit
Before the beneficiary’s first lung cancer LDCT screening, the beneficiary must receive a counseling and shared decision-making visit that meets all of the following criteria, and is appropriately documented in the beneficiary’s medical records:
- Determination of beneficiary eligibility;
- Shared decision-making, including the use of one or more decision aids;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment; and
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions.
Reading Radiologist Eligibility Criteria
For purposes of Medicare coverage of lung cancer screening with LDCT, the reading radiologist must have board certification or board eligibility with the American Board of Radiology or equivalent organization.
Radiology Imaging Facility Eligibility Criteria
For purposes of Medicare coverage, lung cancer screening with LDCT must be furnished in a radiology imaging facility that utilizes a standardized lung nodule identification, classification and reporting system.
The above policy simplifies requirements for the counseling and shared decision-making visit, removes the restriction that it must be furnished by a physician or non-physician practitioner, reduces the eligibility criteria for the reading radiologist, and reduces the radiology imaging facility eligibility criteria (including removes the requirement that facilities participate in a registry). See Appendix B for the expected manual language.
APPENDIX A
General Methodological Principles of Study Design
(Section VI of the Decision Memorandum)
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to that group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where
enthusiasm and psychological factors may lead to an improved perceived
outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is to the extent that differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well-designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of that have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
Randomized controlled trials
Non-randomized controlled trials
Prospective cohort studies
Retrospective case control studies
Cross-sectional studies
Surveillance studies (e. g. , using registries or surveys)
Consecutive case series
Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in that confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to that the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
APPENDIX B
Medicare National Coverage Determinations Manual
Draft
This draft NCD is subject to formal revisions and formatting changes prior to the release of the final NCD contractor instructions and publication in the Medicare National Coverage Determinations Manual.
Table of Contents
(Rev.)
210.14 – Lung Cancer Screening with Low Dose Computed Tomography (LDCT)
A. General
Lung cancer is the third most common cancer and the leading cause of cancer deaths in the United States. Cancer of the lung and bronchus accounted for over 130,000 deaths in 2021 (more than the total number of estimated deaths from colon, breast and prostate cancer combined) with a median age at death of 72 years. Computed tomography (CT) is an imaging procedure that uses specialized x-ray equipment to create detailed pictures of areas inside the body. Low dose computed tomography (LDCT) is a chest CT scan performed at settings to minimize radiation exposure compared to a standard chest CT. Under §1861(ddd) of the Social Security Act (the Act), the Centers for Medicare & Medicaid Services (CMS) has the authority to add coverage of “additional preventive services” through the Medicare national coverage determination (NCD) process if certain statutory requirements are met: (1) reasonable and necessary for the prevention or early detection of illness or disability, (2) recommended with a grade of A or B by the United States Preventive Services Task Force (USPSTF), and (3) appropriate for individuals entitled to benefits under Part A or enrolled under Part B.
B. Nationally Covered Indications
Effective for claims with dates of service on or after 2/10/2022 , CMS has determined that the evidence is sufficient to cover, under Medicare Part B, a lung cancer screening counseling and shared decision-making visit, and for appropriate beneficiaries, annual screening for lung cancer with LDCT, as an additional preventive service benefit under the Medicare program only if all of the following eligibility criteria are met.
Beneficiary Eligibility Criteria
For purposes of Medicare coverage of lung cancer screening with LDCT, beneficiaries must meet all of the following eligibility criteria:
- Age 50 – 77 years;
- Asymptomatic (no signs or symptoms of lung cancer);
- Tobacco smoking history of at least 20 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
- Current smoker or one who has quit smoking within the last 15 years; and
- Receive an order for lung cancer screening with LDCT.
Counseling and Shared Decision-Making Visit
Before the beneficiary’s first lung cancer LDCT screening, the beneficiary must receive a counseling and shared decision-making visit that meets all of the following criteria, and is appropriately documented in the beneficiary’s medical records:
- Determination of beneficiary eligibility;
- Shared decision-making, including the use of one or more decision aids;
- Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment; and
- Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions.
Reading Radiologist Eligibility Criteria
For purposes of Medicare coverage of lung cancer screening with LDCT, the reading radiologist must have board certification or board eligibility with the American Board of Radiology or equivalent organization.
Radiology Imaging Facility Eligibility Criteria
For purposes of Medicare coverage, lung cancer screening with LDCT must be furnished in a radiology imaging facility that utilizes a standardized lung nodule identification, classification and reporting system.
C. Nationally Non-Covered Indications
Preventive services are non-covered by Medicare unless specifically covered in this NCD, any other NCD or in statute or regulations.
D. Other
Medicare Part B coinsurance and deductible are waived for this preventive service.
(This NCD last reviewed 02/10/2022.)
APPENDIX C
Medicare National Coverage Determinations Manual (2014)
210.14 – Lung Cancer Screening with Low Dose Computed Tomography (LDCT) (Effective February 5, 2015) (Rev. 185, Issue: 08-21-15, Effective: 02-05-15, Implementation: 01-04-16)
A. General
Lung cancer is the third most common cancer and the leading cause of cancer deaths in the United States. Cancer of the lung and bronchus accounted for over 150,000 deaths in 2013, with a median age at death of 72 years. Computed tomography (CT) is an imaging procedure that uses specialized x-ray equipment to create detailed pictures of areas inside the body. Low dose computed tomography (LDCT) is a chest CT scan performed at settings to minimize radiation exposure compared to a standard chest CT.
Screening for lung cancer with LDCT is not currently covered under the Medicare program. Under §1861(ddd) of the Social Security Act (the Act), the Centers for Medicare & Medicaid Services (CMS) has the authority to add coverage of “additional preventive services” through the Medicare national coverage determination (NCD) process if certain statutory requirements are met: (1) reasonable and necessary for the prevention or early detection of illness or disability, (2) recommended with a grade of A or B by the United States Preventive Services Task Force (USPSTF), and (3) appropriate for individuals entitled to benefits under Part A or enrolled under Part B.
B. Nationally Covered Indications
Effective for claims with dates of service on or after February 5, 2015, CMS has determined that the evidence is sufficient to add coverage under Medicare Part B a lung cancer screening counseling and shared decision-making visit, and for appropriate beneficiaries, annual screening for lung cancer with LDCT, as an additional preventive service benefit under the Medicare program only if all of the following eligibility criteria are met.
Beneficiary Eligibility Criteria
For purposes of Medicare coverage of lung cancer screening with LDCT, beneficiaries must meet all of the following eligibility criteria:
Age 55 – 77 years;
Asymptomatic (no signs or symptoms of lung cancer);
Tobacco smoking history of at least 30 pack-years (one pack-year = smoking one pack per day for one year; 1 pack = 20 cigarettes);
Current smoker or one who has quit smoking within the last 15 years; and
Receive a written order for lung cancer screening with LDCT. Written orders for lung cancer LDCT screenings must be appropriately documented in the beneficiary’s medical records, and must contain the following information:
Beneficiary date of birth;
Actual pack – year smoking history (number);
Current smoking status, and for former smokers, the number of years since quitting smoking;
Statement that the beneficiary is asymptomatic (no signs or symptoms of lung cancer); and
National Provider Identifier (NPI) of the ordering practitioner.
Counseling and Shared Decision-Making Visit
Before the beneficiary’s first lung cancer LDCT screening, the beneficiary must receive a counseling and shared decision-making visit that meets all of the following criteria, and is appropriately documented in the beneficiary’s medical records:
Must be furnished by a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5) of the Social Security Act), and
Must include all of the following elements:
Determination of beneficiary eligibility including age, absence of signs or symptoms of lung cancer, a specific calculation of cigarette smoking pack-years; and if a former smoker, the number of years since quitting;
Shared decision making, including the use of one or more decision aids, to include benefits and harms of screening, follow-up diagnostic testing, over-diagnosis, false positive rate, and total radiation exposure;
Counseling on the importance of adherence to annual lung cancer LDCT screening, impact of comorbidities and ability or willingness to undergo diagnosis and treatment;
Counseling on the importance of maintaining cigarette smoking abstinence if former smoker; or the importance of smoking cessation if current smoker and, if appropriate, furnishing of information about tobacco cessation interventions; and
If appropriate, the furnishing of a written order for lung cancer screening with LDCT.
Written Orders for Subsequent Annual Lung Cancer Screenings with LDCT
For subsequent annual lung cancer LDCT screenings, the beneficiary must receive a written order for lung cancer LDCT screening. The written order may be furnished during any appropriate visit with a physician (as defined in Section 1861(r)(1) of the Social Security Act) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in Section 1861(aa)(5) of the Social Security Act).
If a physician or qualified non-physician practitioner elects to provide a lung cancer screening counseling and shared decision-making visit before a subsequent annual lung cancer LDCT screening, the visit must meet all of the criteria described above for a counseling and shared decision-making visit.
Reading Radiologist Eligibility Criteria
For purposes of Medicare coverage of lung cancer screening with LDCT, the reading radiologist must meet all of the following eligibility criteria:
Board certification or board eligibility with the American Board of Radiology or equivalent organization;
Documented training in diagnostic radiology and radiation safety;
Involvement in the supervision and interpretation of at least 300 chest computed tomography acquisitions in the past 3 years;
Documented participation in continuing medical education in accordance with current American College of Radiology standards; and
Furnish lung cancer screening with LDCT in a radiology imaging facility that meets the radiology imaging facility eligibility criteria described below.
Radiology Imaging Facility Eligibility Criteria
For purposes of Medicare coverage, lung cancer screening with LDCT must be furnished in a radiology imaging facility that meets all of the following eligibility criteria:
Performs LDCT with volumetric CT dose index (CTDIvol) of ≤ 3.0 mGy (milligray) for standard size patients (defined to be 5’ 7” and approximately 155 pounds) with appropriate reductions in CTDIvol for smaller patients and appropriate increases in CTDIvol for larger patients;
Utilizes a standardized lung nodule identification, classification and reporting system;
Makes available smoking cessation interventions for current smokers; and
Collects and submits data to a CMS-approved registry for each LDCT lung cancer screening performed. The data collected and submitted to a CMS-approved registry must include, at minimum, all of the following elements:
| Data Type |
Minimum Required Data Elements |
| Facility |
Identifier |
| Radiologist(reading) |
National Provider Identifier (NPI) |
| Patient |
Identifier |
| Ordering Practitioner |
National Provider Identifier (NPI) |
| CT scanner |
Manufacturer, Model. |
| Indication |
Lung cancer LDCT screening absence of signs or symptoms of lung cancer |
| System |
Lung nodule identification, classification and reporting system |
| Smoking history |
Current status (current, former, never).
If former smoker, years since quitting.
Pack-years as reported by the ordering practitioner.
For current smokers, smoking cessation interventions available. |
| Effective radiation dose |
CT Dose Index (CTDIvol). |
| Screening |
Screen date
Initial screen or subsequent screen |
Information regarding CMS-approved registries is posted on the CMS website at: http://www.cms.gov/Medicare/Medicare-GeneralInformation/MedicareApprovedFacilitie/Lung-Cancer-Screening-Registries.html.
C. Nationally Non-Covered Indications
Unless specifically covered in this NCD, any other NCD, in statute or regulations, preventive services are non-covered by Medicare.
D. Other
Medicare coinsurance and Part B deductible are waived for this preventive service.
(This NCD last reviewed February 2015.)


