TO: Administrative File: CAG-00449N
FROM: Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group
Joseph Chin, MD, MS
Deputy Director, Coverage and Analysis Group
Lori Ashby, MA
Director, Division of Medical and Surgical Services
Jyme Schafer, MD, MPH
Lead Medical Officer
David Dolan, MBA, MA
Lead Analyst
SUBJECT: National Coverage Determination for Supervised Exercise Therapy (SET) for Symptomatic Peripheral Artery Disease (PAD)
DATE: May 25, 2017
I. Decision
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to cover supervised exercise therapy (SET) for beneficiaries with intermittent claudication (IC) for the treatment of symptomatic peripheral artery disease (PAD). Up to 36 sessions over a 12 week period are covered if all of the following components of a SET program are met:
The SET program must:
- consist of sessions lasting 30-60 minutes comprising a therapeutic exercise-training program for PAD in patients with claudication;
- be conducted in a hospital outpatient setting, or a physician’s office;
- be delivered by qualified auxiliary personnel necessary to ensure benefits exceed harms, and who are trained in exercise therapy for PAD; and
- be under the direct supervision of a physician (as defined in 1861(r)(1)), physician assistant, or nurse practitioner/clinical nurse specialist (as identified in 1861(aa)(5)) who must be trained in both basic and advanced life support techniques.
Beneficiaries must have a face-to-face visit with the physician responsible for PAD treatment to obtain the referral for SET. At this visit, the beneficiary must receive information regarding cardiovascular disease and PAD risk factor reduction, which could include education, counseling, behavioral interventions, and outcome assessments.
- Medicare Administrative Contractors (MACs) have the discretion to cover SET beyond 36 sessions over 12 weeks and may cover an additional 36 sessions over an extended period of time. A second referral is required for these additional sessions.
- SET is non-covered for beneficiaries with absolute contraindications to exercise as determined by their primary physician.
See Appendix B for the NCD manual language.
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms
and corresponding full terminology:
AACVPR - American Association of Cardiovascular and Pulmonary Rehabilitation
ABI - Ankle-Brachial Index
ACC - American College of Cardiology
ACD - Absolute Claudication Distance
ACCF - American College of Cardiology Foundation
ACR - American College of Radiology
AHA - American Heart Association
AHRQ - Agency for Healthcare Research and Quality
APTA - American Physical Therapy Association
ASA - American Stroke Association
AWD - Absolute Walking Distance
CAD - Coronary Artery Disease
CBVD - Cerebrovascular Disease
CCCQ - Charing Cross Claudication Questionnaire
CEPA - Clinical Exercise Physiology Association
CLAU-S - Claudication Scale
CMS - Centers for Medicare & Medicaid Services
COT - Claudication Onset Time
CVC - Cardiovascular Coalition.
ER - Endovascular Revascularization
FCD - Functional Claudication Distance
FDA - Food and Drug Administration
IC - Intermittent Claudication
ICD - Initial Claudication Distance
M - Meters
MEDCAC - Medicare Evidence Development & Coverage Advisory Committee
MNACVPR - Minnesota Association of Cardiovascular and Pulmonary Rehab
MWD - Maximal Walking Distance
MWT - Maximal Walking Time
NCA - National Coverage Analysis
NCD - National Coverage Determination
OMC - Optimal Medical Care
PAD - Peripheral Artery Disease
PAQ - Peripheral Artery Questionnaire
PFWD - Pain Free Walking Distance
PFWT - Pain Free Walking Time
PTA - Percutaneous Transluminal Angioplasty
PWT - Peak Walking Time
QoL - Quality of Life
RCT - Randomized Controlled Trial
SE - Supervised Exercise
SEP - Supervised Exercise Program
SET - Supervised Exercise Therapy
SCAI - Society for Cardiovascular Angiography and Intervention
SIR - Society of Interventional Radiology
ST - Stent Revascularization
SVM - Society for Vascular Medicine
SVS - Society for Vascular Surgery
UE - Unsupervised Exercise
US - United States
VASQoL - Vascular Quality of Life Questionnaire
VIVA - Vascular Interventional Advances
WA - Walking Advice
WIQ - Walking Impairment Questionnaire
CMS initiated this national coverage determination (NCD) to consider coverage under the Medicare program for SET for symptomatic PAD.
The scope of this review is limited to SET for the treatment of symptomatic PAD. Endovascular procedures and medical therapy are not within the scope of this NCA.
PAD
PAD is a vascular disease that stems from atherosclerosis (plaque buildup) which narrows the arteries affecting the lower extremities. The number of people diagnosed with PAD is estimated at more than 200 million worldwide, with approximately 12% of Americans having PAD (Ostchega, Paulose-Ram, Dillon, Gu, & Hughes, 2007; Vun, Miller, Delaney, Allen, & Spark, 2016). The presence of PAD becomes more prevalent with age, with PAD affecting more than 10% of patients in their 60s and 70s (Criqui, 2015). The diagnosis of PAD can be confirmed through the ankle-brachial index (ABI), which is the ratio of systolic pressure at the ankle to that in the arm, or the toe-brachial index for patients where ABI is not reliable due to noncompressible vessels, common for patients of advanced age or chronic diabetes (Anderson et al., 2013; Fowkes et al., 2013). ABI results of 0.91 to 0.99 are considered borderline, with an ABI of 0.90 or less considered abnormal (Anderson et al., 2013).
The most common symptom experienced by people with PAD is IC, which affects about two million Americans, and eight million people globally (Murphy et al., 2012; Vemulapalli et al., 2015). IC is pain/discomfort experienced in the legs that occurs while walking or exercising and resolves itself with rest. The pain from IC often limits PAD patients to very light activity since they are only capable of walking short distances (Hiatt, Regensteiner, Hargarten, Wolfel, & Brass, 1990). This often results in the elimination of many hobbies and work activities, which could have a dramatic impact on patients’ functional independence and quality of life (QoL). Exercise capacity which may be measured by several methods including 6 minute walk test, maximum walking distance, long distance corridor walking and others has been associated with all-cause mortality (Georgiopoulou et al., 2017; Morris et al., 2014; Yazdanyar et al., 2014). While improvement in walking distance and time to claudication is important as a treatment goal, it is also believed that the impact of IC on overall function and general QoL could be minimized.
Treatment for PAD/IC
PAD is an underdiagnosed disease with debilitating consequences that disproportionately affects minority populations. Research has shown SET to be an effective, minimally invasive method to alleviate the most common symptom associated with PAD. This could also prevent the progression of PAD and lower the risk of cardiovascular events that are prevalent in these patients.
Practice guidelines from the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) recommend SET as the initial treatment for patients suffering from IC (Gerhard-Hermanet al., 2016). While experts seem to agree that exercise therapy should be the initial treatment for PAD/IC, the number of endovascular revascularization (ER) procedures has been increasing (Spronk et al., 2008). The preference of physicians and patients for the more invasive ER treatment can be partly attributed to the limited access to SET programs, and the immediate result that is observed with ER (Spronk et al., 2008; van den Houten et al., 2016). ER has remained a more popular treatment option for claudication than SET, despite the ACCF/AHA recommendation that ER be reserved for cases where the patient is too functionally impaired for SET (Anderson et al., 2013).
III. History of Medicare Coverage
CMS does not have an existing NCD specific to SET. While physician-prescribed supervised exercise is covered for Cardiac Rehabilitation (section 1861(eee) of the Social Security Act), coverage is exclusive to cardiac disease. SET for symptomatic PAD has been studied as a separate therapeutic intervention, and not necessarily as a comprehensive program.
A. Current Request
On September 15, 2017, CMS accepted a formal complete request from the American Heart Association to initiate a national coverage analysis (NCA) for SET for symptomatic PAD.
The formal request letter can be viewed via the tracking sheet for this NCA on the CMS website at https://www.cms.gov/medicare-coverage-database/details/nca-tracking-sheet.aspx?NCAId=287.
B. Benefit Category
Medicare is a defined benefit program. For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Social Security Act [§1812 (Scope of Part A); §1832 (Scope of Part B); §1861(s)(1); §1861(s)(2)(A);§1861(s)(2)(B);
SET qualifies as:
- Physicians’ Services
- Incident to a Physician’s Professional Service
- Outpatient Hospital Services Incident to a Physician’s Service
Thus, SET qualifies as a benefit.
IV. Timeline of Recent Activities
| Date |
Action |
| September 15, 2016 |
CMS opens an NCA for Initial 30-day public comment period begins. |
| October 15, 2016 |
First public comment period ends. CMS receives 103 comments |
| March 2, 2017 |
Proposed Decision Memorandum posted. 30-day public comment period begins. |
| April 1, 2017 |
30-day public comment period ends. CMS receives 79 comments. |
V. Food and Drug Administration (FDA) Status
SET services are not subject to FDA regulatory oversight.
VI. General Methodological Principles
When making national coverage determinations under section 1862(a)(1)(A) of the Social Security Act, CMS generally evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. The critical appraisal of the evidence enables us to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for beneficiaries. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
A detailed account of the methodological principles of study design that the Agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A.
Public comments sometimes cite published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information are redacted or will not be made available to the public. CMS responds in detail to the public comments on a proposed national coverage determination when issuing the final national coverage determination.
VII. Evidence
A. Introduction
The evidence reviewed to date includes the published medical literature on pertinent clinical trials of SET. The aim of therapies for IC from PAD is relief of symptoms, overall cardiovascular risk reduction, avoidance of amputation, and preservation of walking and functional status. The most commonly reported outcome measures are walking parameters which include initial claudication distance (ICD), similar to pain-free walking distance (PFWD), and absolute claudication distance (ACD), which can also be called maximal walking distance (MWD). These measures have been shown to reflect functional capacity in patients with IC. Another measure is functional claudication distance (FCD), the distance a patient prefers to stop, and this measure also has been shown to be reliable and valid (Kruidenier 2009). Six- minute walk test with a standardized protocol is sometimes reported, as is pain-free walking time and maximal walking time.
Clinical trial outcome measures also include QoL and function questionnaires, amputations, mortality, other cardiovascular events, and ABI. A number of disease-specific QoL assessments for intermittent claudication are available for use (Mehta 2003). One of the more popular questionnaires is the Walking Impairment Questionnaire (WIQ). It was developed and validated to quantify the ability to walk defined distances, speeds, and stairs and to characterize the symptoms that may limit ability to walk in patients with IC. The short physical performance battery is designed to assess lower extremity function and combines data from 4-meter walking velocity, time to rise and stand from a seated position five times, and standing balance. Others include the Charing Cross Claudication Questionnaire (CCCQ), a patient administered questionnaire that provides a quantitative measure of improvement in symptoms, the Claudication Scale (CLAU-S), and the Vascular Quality of Life (VascQoL) (Morgan 2001). Questionnaires such as the SF-36 are also used to evaluate general health and well-being, though when looking at these scores it is reflective of overall well-being.
B. Discussion of Evidence
1. Evidence Question(s)
Is the evidence sufficient to conclude that SET improves health outcomes for Medicare beneficiaries with intermittent claudication due to symptomatic PAD?
If the answer to the question above is positive, is the available evidence sufficient to identify program characteristics that are more likely to lead to overall benefit from SET?
2. External Technology Assessments
CMS requested an external technology assessment (TA) on this issue for MEDCAC presentation.
Relative effectiveness of exercise therapy
Jones WS, Schmit KM, Vemulapalli S, Subherwal S, Patel MR, Hasselblad V, Heidenfelder BL, Chobot MM, Posey R, Wing L, Sanders GD, Dolor RJ. Treatment strategies for patients with peripheral artery disease. Comparative Effectiveness Review No. 118. (Prepared by the Duke Evidence-based Practice Center under contract No. 290-2007-10066-I). AHRQ Publication No. 13-EHC090-EF. Rockville, MD: Agency for Healthcare Research and Quality; May 2013. Available at: https://www.ncbi.nlm.nih.gov/books/NBK148574/.
This AHRQ sponsored study was presented at the July 2015 Lower Extremity PAD MEDCAC. This report compared strategies including exercise training versus usual care, endovascular intervention versus exercise training, and surgical revascularization versus exercise and medical therapy, and did a fixed effect model looking at the comparisons, and a network meta-analysis comparing each against each other. Findings of the report included:
- SET and the combination of ER + exercise training resulted in large improvements in MWD in adults with IC (when compared with usual care). The average age of participants for studies of SET versus usual care was 63 years to 76 years. Strength of Evidence: Moderate.
- A network meta-analysis found no individual treatment (exercise training, cilostazol, endovascular intervention) to have a statistically significant effect when compared to others for adults with IC with MWD or ACD as an outcome.
- Exercise training was found to have moderate to large effects on ICD/PFWD. Strength of Evidence: Low.
- A network meta-analysis found no individual treatment (cilostazol, exercise training, endovascular intervention) to have statistically significant effect when compared to others for adults with IC with ICD/PFWD as an outcome.
- Exercise training was found to have moderate to large effects on QoL when compared with usual care. Strength of Evidence: Low
- A network meta-analysis found no individual treatment (cilostazol, exercise training, endovascular, surgical) to have statistically significant effect when compared to others for adults with IC with QoL as the outcome.
- Inconclusive evidence for exercise training (and cilostazol and ER) in IC for nonfatal MI, nonfatal stroke, amputation, and general safety. Strength of Evidence: Insufficient.
- There were no studies for exercise training (and cilostazol and ER) in IC for composite cardiovascular events, wound healing, pain, and safety in subgroups. Strength of Evidence: Insufficient.
Presented data at the MEDCAC included publications since the 2013 AHRQ review and included the 18 month CLEVER trial results (Murphy et al. 2014).
National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease: Diagnosis and management. NICE Clinical Guideline 147. Methods, evidence and recommendations August 2012. https://www.nice.org.uk/guidance/cg147/evidence/lower-limb-peripheral-arterial-disease-full-guideline-186865021
An analysis was undertaken examining supervised exercise (SE) for the treatment of PAD in adults with IC. Evidence tables in the 2012 report include:
- The characteristics of the twelve studies that met inclusion criteria to compare SE to unsupervised exercise (UE) are listed in Table 27.
- Tables 28 and 29 are clinical evidence profiles of these studies.
- Table 30 lists reason for withdrawal from exercise programs when available from included studies.
- Tables 31 and 32 are QoL data.
Modeling included estimations of baseline mortality and relative risk associated with exercise, baseline risk of cardiovascular events and relative risk associated with exercise, short and long term compliance, and probabilistic simulation in the change of QoL over time.
Absolute change in MWD and QoL were considered to be the most important outcomes in measuring the success of exercise therapy. Though none of the studies reported data on cardiovascular events or limb loss, these outcomes were felt to be of more importance in critical limb ischemia than IC.
A consensus on the features of an exercise program including the following:
- Patients should be encouraged to walk to the point of maximal pain.
- The frequency of the program should be approximately 2 hours per week for 3 months.
- The program should have goals and a defined educational component. Discussions should include lifestyle change, benefits of exercise and attitudes to the disease.
- A qualified healthcare professional should be in charge of the program.
- The location should be as close to a patient’s home as possible.
Other analyses included supervised exercise as a part of combination therapy.
The 2012 evidence review concluded that in the management of IC, offering a SEP is the only recommendation identified as a priority for implementation.
National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease Evidence Update November 2014. A summary of selected new evidence relevant to NICE clinical guideline 147 ‘Lower limb peripheral arterial disease: diagnosis and management’ (2012) https://www.nice.org.uk/guidance/cg147/evidence/evidence-update-186861133
Key points in the 2014 clinical evidence update included:
“Management of intermittent claudication
Exercise programmes
- Supervised exercise is associated with increases in MWD compared with home-based or other unsupervised exercise programmes.
- Supervised exercise is associated with greater increases in walking distance in people with aorto-iliac disease than either stenting or optimum medical care.
- Supervised exercise appears to be more cost effective than either angioplasty alone or supervised exercise plus angioplasty in people with IC due to femoro-popliteal occlusion."
Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA 1995;274:975-980.
The purpose of this meta-analysis was to identify the components of an exercise rehabilitation program that were most effective in improving the symptoms of IC. Studies were included if walking distance or time to onset of claudication pain and to maximal pain during a treadmill test were reported. Both randomized and non-randomized studies were included. Data abstraction and analysis are described. Twenty one studies met inclusion criteria. Studies varied in their exercise duration, exercise component, frequency program length, and program location. No study reported the intensity of exercise and few reported attendance, making it difficult to further quantify how much exercise was performed. Length of the programs ranged from 4 to 52 weeks, classes per week ranged from 2 to 10. Distances to onset of claudication pain increased from (mean ±± SD) 125.9 ± 57.3 m to 351.2 ± 188.7 m and the distance to maximal claudication pain increased from 325.8 ± 148.1 m to 723.3 591.5 m. Greatest improvements in distances were associated with these program variables: duration greater than 30 minutes per session, frequency of at least three sessions per week, walking used as the mode of exercise, use of near maximal pain as an end point, and program length of greater than 6 months. In a post hoc analysis, age (62.8 ± 3.3 years, range 57.9 to 68.0 years) was the only characteristic that significantly correlated with change in distance to onset and maximal claudication pain, suggesting that older patients may derive more benefit than younger patients. The authors conclude, “The optimal exercise program for improving claudication pain distances in patients with peripheral arterial disease uses intermittent walking to near-maximal pain during a program of at least 6 months.”
Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2014. Issue7. Art. No.:CD000990. doi: 10.1002/14651858.CD000990.pub3.
The purpose of this review was to determine if an exercise program in patients with IC was effective in treating symptoms and increasing treadmill walking times and distances. This systematic review with meta-analysis included 30 RCTS with 1816 participants. Trials were of an exercise program versus control or medical therapy in patients with IC. All exercise program studies except one included some element of supervision. Studies were excluded if the control included walking advice as usual care/best medical treatment. Inclusion of trials was not affected by the duration, frequency or intensity and any type of exercise was included. Outcome measures must have had any of these measures: treadmill walking distance measures (such as PFWD, PFWT, MWT, MWD); ABI; QoL; morbidity or amputation. Trial length varied from 2 weeks to 2 years. Compliance with exercise was variable. While type of exercise varied, most trials used a treadmill walking test for one of the outcome measures. Most trials were small with 20 to 49 participants. Mean age of included trials appeared to be between 65 and 67 years, with inclusion in many trials of people in the 70s and some in their 80s. The quality of the trials was determined to be moderate due mainly to lack of information. Maximal walking time compared with usual care improved 4.5 minutes (95% CI 3.11 to 5.92) on average. PFWD increased by 82.29 meters (95% CI 71.86 to 92.72) and MWD increased by 108.99 meters (95% CI 38.20 to 179.78). Improvements were seen up to two years. ABI was not shown to improve. Data on mortality and amputation were inconclusive and there was no data on non-fatal cardiovascular events. The authors concluded that exercise programs clearly improve walking time and distance for people who were considered able to participate in exercise programs, with a benefit demonstrated up to two years.
Frans FA, Bipat S, Reekers JA, Legemate DA, Koelemay MJ. Systematic review of exercise training or percutaneous transluminal angioplasty for intermittent claudication. Br J of Surg. 2012 Jan;99(1):16-28. doi:10.1002/bjs.7656.
The aim of this systematic review was to summarize the results of all RCTs comparing percutaneous transluminal angioplasty (PTA) with SET in patients with IC to determine relative effectiveness. Study inclusion and exclusion criteria are listed. Eight RCTs were included. The methodological quality of the included studies was assessed using the Cochrane checklist, with the overall quality being mediocre and one study of high quality. Study populations, interventions, and outcome assessment were heterogeneous and precluded meta-analysis. Exercise interventions varied from home-based exercise to SET programs. Judging from their results, the authors felt the effectiveness of PTA and
exercise therapy to be equivalent, but combined therapies may be a further improvement. The authors conclude, “A combination of PTA and exercise (SET or ET advice) may be superior to exercise or PTA alone, but this needs to be confirmed.”
Effectiveness of supervised versus unsupervised exercise
Vemulapalli S, Dolor RJ, Hasselblad V, Schmit K, Banks A, Heidenfelder B, Patel MR, Jones WS. Supervised vs unsupervised exercise for intermittent claudication: A systematic review and meta-analysis. Am Heart J. 2015 Jun;169(6):924-937.e3. doi: 10.1016/j.ahj.2015.03.009 PMID:26027632
This systematic review and meta-analysis included 28 articles from 27 unique studies (24 RCTs and 4 observational studies) with 2,074 patients that evaluated the comparative effectiveness of SE with UE. Studies were included based on the stated inclusion parameters. Outcomes assessed were walking parameters, claudication parameters, patient-reported outcomes (from SF-36, peripheral artery questionnaire, and WIQ). Documentation of exercise compliance was not used as an inclusion criterion as most studies do not report this. Improved compliance is one of the potential mechanisms of benefit for SE as compared to UE. Meta-analysis was considered for RCTs with comparisons where at least 3 studies reported the same outcome. Random-effects models were used for outcomes due to the heterogeneity of the studies. Effect size interpretation was based on Hedge g, where 0 is no effect, 0.2 is a small effect, 0.5 is a medium effect, 0.8 is a large effect, and effects larger than 1.0 are very large effects. The authors report statistical heterogeneity between studies. Of the 27 studies, 25 reported maximal walking measures (13 reported 6 month and 4 reported 12 month), 25 studies reported claudication measures, (14 met inclusion for 6 months and 3 met inclusion for 12 months), 13 studies reported QoL (5 met inclusion at 3 months and 4 met inclusion at 6 months), and four studies reported WIQ speed and distance and met inclusion at 3 months. Of the studies reporting maximal walking measure, the maximal walking distance for SE compared to UE improved 118 m (95% CI 55 – 179 m, P < 0.001) at 6 months and 86 m (95% CI 52 – 118 m, P < 0.001) at 12 months. Of the studies reporting claudication measures, claudication distance improved 35 m (95% CI 22 - 48 m, P < 0.001) at 6 months and 23 m (95% CI 10 - 36 m, P < 0.001) at 12 months for SE compared with UE. There was no statistical difference between SE and UE in SF-36 physical functioning QoL at 3 months and 6 months. There was no difference in WIQ distance or speed at 3 months between SE and UE. A sensitivity analysis was conducted restricting studies to those with good quality and in which SE was performed at least 3 times per week for at least 12 weeks (per AHA guidelines). Using this group of studies, MWD, PWT, or ACD at 6 months, 4 studies showed a large effect size. For COT, PFWD, or ICD at 6 months, 4 studies showed a large effect size. Publication bias was assessed as low. The authors concluded, “In claudication patients, SE is more effective than UE at improving maximal walking and claudication distances, yet there is no difference in general quality of life or patient-reported community-based walking. Further studies are needed to investigate the relationship between functional gain and disease-specific quality of life.”
Fokkenrood HJP, Bendermacher BLW, Lauret GJ, Willigendael EM, Prins MH, Teijink JAW. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.:CD005263. doi: 10.1002/14651858.CD005263.pub3.
This systematic review with meta-analysis is an update of a 2006 review. It included 14 RCTs with 1002 patients with a mean age of 67 years, with trial size ranging from 20 to 304. Trial participants included patients with IC due to atherosclerotic disease diagnosed by questionnaire or clinically. Trial length ranged from six weeks (minimum criteria for trial length) to 12 months. While programs differed, in general the supervised program consisted of three sessions per week. Inclusion of trials was not limited by frequency or intensity of the program. The study control of non-supervised exercise was defined as walking advice or a structured home-based exercise program. Studies with control groups that did not receive exercise or walking advice or received usual care were excluded. All trials had treadmill walking in distance or time as an outcome, with baseline data available. The quality of the trials was judged as moderate to good. Before the program, walking distance was around 300 meters, with a pain-free distance of 200 meters. An overall effect size of 0.69 (95%CI 0.51 to 0.86) at 3 months and 0.48 (95% CI 0.32 to 0.64) at six months was found for SET in comparison to UE, which is a difference of about 180 meters. Benefit for maximal and pain-free walking distance continued to 12 months. There was no statistical difference in QoL measurement, with the authors suggesting that this could be due to lack of power. Thirteen of the participants died but none were related to exercise therapy. The authors noted two important limitations in the quality of evidence: blinding and participation bias. Funnel plots suggest that publication bias was not important. The authors conclude that supervised exercise therapy provides statistically significant benefits for treadmill walking distance when compared with non-supervised programs and commented that additional studies are needed to focus on QoL and other functional outcomes.
Wind J, Koelemay MJW. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomized controlled trials. Eur J Vasc Endovasc Surg 2007 Jul;34(1):1-9. doi: 10.1016/j.ejvs.2006.12.030
This systematic review examined the additional benefit of supervision to exercise therapy for intermittent claudication. Included in the analysis are 15 randomized controlled trials published between 1990 and 2006 and reported on 761 patients, with a range of 14 to 149 patients per study. Age summary for the individual studies ranges from 60 years to 76 years. Most of the programs lasted 12 to 26 weeks with a frequency of 2 to 3 times per week. One supervised program lasted 2 years. Dropout rates ranged from 0 to 50 in either group. Most dropouts were related to medical reasons or refusal by the patient to continue in the program. Similar drop-outs were reported in the control group. Few patients experienced adverse effects. The overall methodologic quality was judged as moderate. Results of the review are presented below.
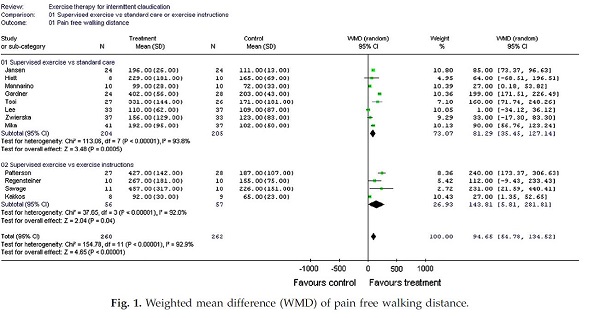
Page 7. Figure 1. Weighted mean difference (WMD) of pain free walking distance. Wind J and Koelemay MJW. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomized controlled trials. Eur J Vasc Endovasc Surg 2007 Jul;34(1):1-9.
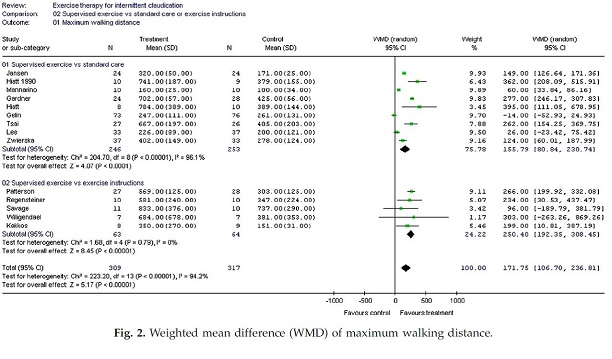
Page 7. Figure 2. Weighted mean difference (WMD) of maximum walking distance. Wind J and Koelemay MJW. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomized controlled trials. Eur J Vasc Endovasc Surg 2007 Jul;34(1):1-9.
In summary, the studies comparing SET to standard care, the weighted mean difference in PFWD and absolute walking distance (AWD) was 81.3 meters (95% CI; 35.5 – 127.1) and 155.8 meters (95% CI; 80.8 – 230.7). Comparing supervised to unsupervised exercise therapy, the weighted mean difference in PFWD and AWD was 143.8 m (95% CI; 5.8 – 281.8) and 250.4 meters (95% CI; 192.4-308.5). The authors concluded that exercise therapy increases the PFWD and AWD in patients with intermittent claudication but noted that the additional value of supervision over unsupervised exercise needs further clarification.
Gommans LNM, Saarloos R, Scheltinga MRM, Houterman S, De Bie RA, Fokkenrood HJP, Teijink JAW. Editor’s Choice – The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. J Vasc and Endovasc Surg 2014 Aug;48(2):169-184. doi:10.016/j.ejvs.2014.04.019.
This systemic review examined the effect of supervision on walking capacity in patients with IC with the hypothesis that there is a dose-response type effect between the intensity of supervision and improvement in walking. Criteria for study inclusion is provided. Thirty studies involving 1406 patients (68% men; mean age 68 years) with IC were included. The overall study quality was noted to be moderate to good. The number of patients in included trials varied from 20 to 304. Treatments were categorized by the type of supervision.
- Control group (NO-ET): Walking advice was not provided.
- Walking advice (WA): Patients were advised to increase activity levels by walking but supervision and monitoring were absent.
- Home-based ET (HB-ET): Patients were advised to increase physical activity which was both self-monitored and actively monitored by a physician, nurse, or physiotherapist a maximum of twice a week.
- SET: Patients received a supervised program either with or without additional walking advice. The program was under supervision of trained medical personnel and consisted of at least 2 supervised sessions per week.
All trials used SET as a comparator except one. A sensitivity analysis was performed. The 6 month comparison results for MWD and PFWD as standardized mean difference for the four groups are presented graphically below.
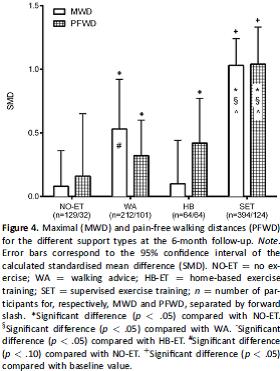
Figure 4. Page 179. Gommans LNM, Saarloow R, Scheltinga MRM, Houterman S, De Bie RA, Fokkenrood HJP, Teijink JAW. Editor’s Choice – The effect of supervision on walking distance in patients with intermittent claudication: a meta-analysis. J Vasc and Endovasc Surg 2014 Aug;48(2):169-184.
The authors conclude that the intensity of supervision is related to improved walking distance and that supervised exercise therapy for IC is superior to all other forms of exercise therapy.
Safety of supervised exercise
Gommans LNM, Fokkenrood HJP, van Dalen HCW, Scheltinga MRM, Teijink JAW, Peters RJG. Safety of supervised exercise therapy in patients with intermittent claudication. J Vasc Surg 2015;Feb;61(2):512-518. doi: 10.1016/j.jvs.2014.08.070.
This systematic review examined the safety of supervised exercise therapy. Search strategy, types of studies, data extraction and management were provided. Seventy-four studies were included for a total of 2,876 participants with IC, with 82,725 patient-hours of SET. The mean age was 64 (range of 54 to 76 years) with 71% men. The usefulness of cardiac screening before SET was evaluated in a sub-analysis. Potential adverse events were explicitly reported in 35 of 74 studies with another 32 studies reporting either no drop outs or no drop outs for medical causes. There were 8 reported adverse events:
| Event |
No. |
| Heart arrhythmia |
1 |
| Angina |
2 |
| Cardiac arrest (successfully resuscitated) |
1 |
| Myocardial infarction (fatal) |
1 |
| Increased heart rate and dyspnea |
1 |
| Worsening of osteoarthritis |
1 |
| Ischialgia (no treatment needed) |
1 |
The total all-cause event rate was one event per 10,340 patient hours. Total cardiac and noncardiac event rates were one per 13,788 and one per 41,363 patient-hours. The authors note a methodological concern that in some studies vulnerable cardiac patients were excluded, which would result in an underestimation of adverse events. However, at the same time exercise reduces all-cause mortality, so as a consequence patients with cardiac comorbidity should not be routinely excluded but rather carefully monitored. The authors conclude that SET can be safely prescribed in patients with IC and that routine cardiac screening before starting SET is not required.
3. Internal Technology Assessment
Literature Search Methods
In addition to the evidence submitted by the American Heart Association, CMS searched PubMed for publications in English from January 1995 to October 2016. Search terms included the following: claudication AND exercise, and included clinical trials, comparative studies, meta-analysis, observational studies, pragmatic clinical trials and systematic reviews (excluding economic studies), with a trial size at a minimum of 10 participants per arm. CMS notes that there are trials that date decades before 1995; the date of 1995 coincides with the publication of the meta-analysis in JAMA by Gardner. A total of 663 titles were found and abstracts were reviewed for relevancy dating back ten years (January 2007). These abstracts (69) were further reviewed and compared to the requestor’s submission to ascertain if additional evidence would add or subtract from the evidence base submitted by the requestor. In summary, the totality of published evidence is large and not all studies are included in this review, however the broad conclusion of studies appears to be similar. We have focused on Cochrane reviews, systematic reviews, randomized controlled trials and large observational studies. Systematic reviews and meta-analyses are listed in the external TA section and evidence tables are available in the respective articles. The requestor provided a number of published RCTs and observational studies; listed below are those trials published since 1995 with at least 20 participants. CMS recognizes the importance of the earlier trials and all individual studies and systematic reviews to contribute to the evidence base for this intervention.
Randomized Controlled Trials
Therapy comparison and extended follow-up
Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Jaff MR, Comerota AJ, Steffes MW, Abrahamsen IH, Goldenberg S, Hirsch AT. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: The CLEVER study. JACC 2015 MAR; 65(10):999-1009. doi: 10.1016/j.jacc.2014.12.043.
This NIH sponsored randomized controlled multicenter trial compared medical therapy (OMC) with home exercise, OMC plus SE or OMC plus stent revascularization (ST) in patients with moderate to severe claudication due to aortoiliac PAD. One hundred and eleven patients were randomized, with 79 completing the 18 month clinical and treadmill assessment. Mean age of those enrolled was 64.4 years ± 9.5 with 62.2% male participants, with many comorbidities. SE consisted of a 6 month program (treadmill walking three times a week) with routine telephone counseling for an additional year, and also received OMC. OMC consisted of cilostazol, home exercise counseling, and atherosclerosis risk factor management. The primary endpoint was peak walking time (PWT) on a graded treadmill test. Secondary endpoints included QoL with the Peripheral Artery Questionnaire (PAQ), the SF-12, and the WIQ. Patient disposition is provided. Among SE participants 71% attended at least 70% of their scheduled sessions. Six month results showed improved walking times for both SE (5.8 min ± 4.6 min) and ST (3.7 min ± 4.9 min), and the ST arm having a greater increase in QoL. At 18 months, PWT improved from baseline for both SE (for 32 of 43 patients, 5.0 min ± 5.4 min) and ST (for 41 of 46 patients, 3.2 min ± 4.7 min) as compared to OMC (for 18 of 22 patients, 0.2 min ± 2.1 min). The difference between SE and ST was not significant. At 18 months, PAQ was statistically better in the ST group than SE or OMC, though for the SF-12 physical and WIQ there was no significant difference between the SE group and the ST group. There were 3 major adverse events: a myocardial infarction in the OMC group, a death in the SE group, and a target limb revascularization in the ST group. Four stent procedure-related adverse events occurred in 3 of the ST participants. The authors concluded that SE and ST provide comparable durable improvement in functional status and in QoL up to 18 months.
Fakhry F, Rouwet EV, den Hoed PT, Hunink MGM, Spronk S. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J of Surg 2013;100:1164-1171. DOI 10.1002/bjs.9207.
This study is the longer term follow-up of the randomized controlled Comparing Exercise Therapy with Angioplasty for Claudication (CETAC) trial. In this trial 151 patients with IC due to aortoiliac or femoropopliteal disease were randomized to SET or ER as initial treatments. Outcome measures included PFWD and MWD, SF-36, the VascuQoL and the number of secondary interventions. The SET program consisted of 24 weeks of supervised treadmill exercise, 2 sessions per week for 30 minutes. Patients were encouraged to walk at home. The SET group adherence was good with a mean attendance of 33 sessions. After a median follow-up of 7 years (range 0.07 to 9.17 years), 17 patients in the SET group and 15 in the ER group had died. Thirty-six patients were available for review in the SET group and 47 in the ER group. The mean long-term improvement in the SET group was 975m (95% CI 772 – 1177) for MWD and 700 m (95% CI 461 – 941) for PFWD. Both SET and ER groups had improved VascuQoL and there was no significant difference between groups. There were no significant differences in the SF-36 scores between groups. The secondary intervention rate was higher in the SET group, however the total number of invasive interventions were higher in the ER group. The authors conclude “In the longer term, SET-first or ER-first treatment strategies were equally effective in improving functional performance and QoL in patients with intermittent claudication.”
Cardiovascular measurements
Hodges LD, Sandercock GRH, Das SK, Brodie DA. Randomized controlled trial of supervised exercise to evaluate changes in cardiac function in patients with peripheral atherosclerotic disease. Clinical Physiology and Functional Imaging 2008 Jan; 28(1):32-37. DOI: 10.1111/j.145-097x.2007.00770.x
Twenty eight patients with IC were randomized to either SET or UE after peak oxygen consumption was assessed with a graded treadmill test. Mean age was 68 ± 8 years. The SET consisted of twice weekly visits for 12 weeks. Patients in the SET group were encouraged to walk on a treadmill until they reached certain levels of claudication pain, which was repeated until each patient had accrued 30 minutes of exercise per session. After 12 weeks there was a significant improvement (91%) in MWD following SET but no significant changes in peak oxygen consumption, peak cardiac output, peak heart rate, or peak cardiac power outcome, but they were able to complete a higher workload for equivalent demands on the circulatory system. The authors conclude that a short-term period of SET results in improved walking time in patients when walking is limited by claudication, and that the cardiovascular system becomes more efficient in meeting the demands of exercise.
Short term intensive exercise
Gibellini R, Fanello M, Bardile AF, Salerno M, Alio T. Exercise training in intermittent claudication. International Angiology 2000 Mar; 19(1):8-13.
This study sought to analyze the effect of short term intensive exercise therapy. Forty patients (36 male and 4 female, mean age 66.5 years) with IC were enrolled in the study and randomized. Twenty patients had supervised intensive exercise for 4 weeks and 20 formed the control group. Baseline data included treadmill testing and ABI. The exercise program consisted of 30 minutes of treadmill work twice a day for 5 days a week, for 4 weeks. The control was described as non-exercising. Patients were re-evaluated at 4 weeks and 6 months. In the treatment group, 2 patients were asymptomatic at 4 weeks and 3 patients were asymptomatic at 6 months. The remainder of the group increased their initial claudication distance at 4 weeks (127.8 to 308.2 m) and 6 months (116.8 to 351.4 m). Maximal claudication distance increased as well at 4 weeks (8 were asymptomatic, the remainder went from 217 to 450 m) and was maintained at 6 months (11 were asymptomatic, the remainder went from 203 to 393.6 m). There was no statistical difference in the control subject’s claudication distances. The authors conclude, “The rehabilitative approach, which also includes information and education strategies, allows the patient to make a concrete change in life-style thus maintaining the results in the long-term.”
Exercise in disabled older adults
Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hockberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: A randomized controlled trial. J Am Geriatr Soc 2001 Jun;49(6):755-762.
The objective of this RCT was to determine the effects of a 6-month exercise program on ambulatory function, physical activity, peripheral circulation, and QoL in disabled older patients with IC. Non-smoking patients were recruited from advertisements and after screening, a comprehensive medical evaluation was given. Sixty-one patients were enrolled and randomized to either exercise rehabilitation (31) or usual care (30). Three patients from the exercise group and six patients from the control group dropped out, leaving 28 patients (age 71 years ± 1, 46% Caucasian) in the treatment group and 24 (age 70 ± 1, 67% Caucasian) in the control. The group was highly comorbid. The control group was usual care, non-exercising. Measurements included treadmill distance walked to onset of claudication and maximal claudication, 6-minute walk test, the WIQ, perceived QoL (SF-36), and daily physical activity (accelerometer and questionnaires). The exercise program consisted of 6 months of supervised, intermittent treadmill walking to near maximal claudication pain 3 days per week. There were no complications during training sessions. Compliance with exercise sessions was 73% with 19 of 28 patients attending at least 70% of the sessions. The exercise group in comparison to the control group increased treadmill distance walked to initial claudication (172 m ± 26 to 402 m ± 56 as compared to 163 m ± 23 to 203 m ± 43), and to maximal claudication (396 m ± 43 to 702 m ± 57 as compared to 379 m ± 48 to 425 m ± 56), and accelerometer-derived physical activity. There was no change in the WIQ and SF-36, for which the authors suggest several reasons (not measuring the increase in type of activity, ambulatory dysfunction only one of many factors influencing self-perceived health related QoL, QoL may lag behind improvement in ambulatory function). The authors concluded “Improvement in treadmill claudication distances in these patients translated into increased accelerometer derived physical activity in the community, which enabled the patients to become more functionally independent.”
Extended follow-up in older adults
Gardner A, Kaztle LI, Sorkin JS, Goldberg AP. Effects of long-term exercise rehabilitation on claudication distances in patients with peripheral arterial disease: A randomized controlled trial. Journal of Cariopulmonary Rehabilitation. 2002 May/Jun;22(3):192-198.
This trial is a continuation of the Gardner 2001 trial and was done to examine if the effects of SET could be sustained with an extended maintenance program in older adults. Seventeen patients (mean age 72 years) continued in the progressive exercise program two times per week for the next 12 months of a maintenance program and a control of 14 patients (mean age of 71 years) that did not receive any recommendations regarding exercise were also followed. At the 18 month evaluation improvements in the SET group were maintained and were significantly different than the control group. The authors concluded “The major findings of the investigation were that an 18-month program of exercise rehabilitation improved ICD by 189%, ACD by 80%, walking economy by 11%, 6 minute walk distance by 10%, physical activity level by 31%, and peripheral circulation by up to 30%. These changes were similar to those obtained after the first 6 months of exercise rehabilitation even though the frequency of the exercise program was reduced from three sessions per week during the first 6 months to two sessions per week during the final 12 months.”
Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication: a randomized controlled trial. J Vasc Surg 2012 May; 55(5): 1346 – 1354. Doi:10.1016/j.jvs.2011.11.123
The goal of this trial was to examine optimal supervised exercise program length to efficaciously change COT and PWT. Patients were recruited from the Vascular Clinic at a Veterans Affairs Health Care System and from advertisements. Power calculations were done to estimate sample sizes to achieve 80% power and allow for 30% drop out rate. Medication regimen of each patient was held constant during the study. Inclusion criteria included ABI and exclusion criteria are listed in the article. One hundred forty-two patients were randomized 3:1 to either supervised exercise (n=106) or usual care (n=36), with a mean age of 68 years and a number of comorbidities. The exercise program consisted of progressive intermittent walking to near maximal claudication pain three days per week and the trial lasted for 6 months. The control group was encouraged to walk but were not given a specific program. COT and PWT were the primary outcomes and were obtained from a treadmill test obtained at baseline, 2, 4, and 6 months. The program was supervised by exercise physiologists and nurses. Twenty six in the exercise group and 9 in the control group did not finish the study. The primary reasons were lack of interest and medical events. Adherence to exercise declined after 2 months with patients completing on average 53 ± 24 sessions of 72 scheduled. Primary outcomes:
Table 1. Treadmill test exercise measures (mean, SD) of patients in exercise group (n=106) and control group (n=36)
| Variable |
Baseline |
Month 2 |
Month 4 |
Month 6 |
| Claudication Onset Time (sec) |
|
|
|
|
| Control Group |
146 (112) |
225 (136)* |
213 (158)*^ |
218 (159)* |
| Exercise Group |
189 (142) |
333 (188)* |
382 (210)*^ |
411 (232) *^ |
| Peak Walking Time (sec) |
|
|
|
|
| Control Group |
386 (226) |
469 (155) |
436 (236) |
446 (308) |
| Exercise Group |
431 (243) |
651 (267)* |
711 (286)*^ |
746 (297)*^ |
*change from baseline (p < 0.05)
^change from Month 2 (p < 0.05)
The authors concluded “Exercise-mediated gains in COT and PWT occur rapidly within the first two months of exercise rehabilitation, and are maintained with further training.”
Gender effects
Gommans LNM, Scheltinga MRM, Van Sambeek MRHM, Maas AHEM, Bendermacher BLW, Teijink JAW. Gender differences following supervised exercise therapy in patients with intermittent claudication. J Vasc Surg 2015 Sep;62(3):681-688.
This study is an analysis of a subset of the 2010 EXITPAD data to examine the relationship of gender differences following SET in patients with IC. This analysis included 113 men and 56 women. In the two groups, the men had a mean age of 65 years (SD 9.2) and the women had a mean age of 67 years (SD 9.7). At baseline, groups were similar in terms of clinical characteristics and ACD (men 250 meters; women 270 meters). ACD improved for both men and women, but the increase for women was less at 3 months (280 meters improvement for men versus 220 meters for women) and ACD was shorter for women compared with men after one year (565 meters versus 660 meters). Total WIQ scores were similar as was QoL. The authors concluded “Women with IC benefit less during the first 3 months of SET and have lower absolute walking distances after 12 months of follow-up compared with men. More research is needed to determine whether gender-based IC treatment strategies are required.”
Functional status and exercise type
Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg 1996;23(1):104-115.
This trial examined functional status and different types of exercise. Twenty-nine male patients (average age 67) were randomized to one of three groups: 10 were in supervised treadmill training (3 hours per week of progressive training by a physical therapist), 9 were in strength training (3 hours per week of leg muscle work), and 10 were in the non-exercising control. Treadmill PWT, the Physical Activity Recall (PAR), SF-20, the WIQ, and an activity monitor were used as measurements. At 12 weeks there was some cross-over. The treadmill and strength training groups did 12 more weeks of treadmill work and the control group did a combination of treadmill and strength training. The trial ended at 24 weeks. Results showed the 24 week treadmill group to have the most improvement (treadmill group, mean (SD): baseline of 9.6 min ± 5.7 to 17.2 min ± 7.3; strength then treadmill group, mean (SD): baseline of 6.5 min ± 2.9 to 11.8 min ± 6.0; control then treadmill/strength group, mean (SD): baseline of 7.4 min ± 3.3 to 13.2 min ± 6.6) with improved PAR, SF-20 scores, and WIQ scores as well as increased activity via the activity monitor. The authors concluded that treadmill training alone was most effective at improving functional status in patients with IC as compared to strength training or a combination of exercise.
SET outcomes with ABI criteria for study entry
McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, Kibbe M, Domanchuk K, Stein JH, Liao Y, Tao H, Green D, Pearce WH, Schneider JR, McPherson D, Laing ST, McCarthy WJ, Shroff A, Criqui Mh. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication. JAMA 2009;30(2):165-174.
This NIH funded trial examined if either supervised treadmill exercise or lower extremity resistance training improve functional performance of patients with PAD with and without classical symptoms of claudication. ABI was the main qualifying inclusion criteria. A total of 156 patients with PAD were randomly assigned to supervised treadmill therapy (3 times a week for 24 weeks), lower extremity resistance training (3 times a week for 24 weeks), or to a control group. Randomized patients had an average age of 73, included 52% women and 39.7% blacks. The primary outcome measures were six-minute walk and the short physical performance battery. Secondary measure included treadmill walking performance, the WIQ, and SF-36. Randomization, power calculations, patient recruitment and follow-up are fully described. The supervised exercise group improved their distance by 35.9 m (95% CI 15.3 – 56.5m) compared to the control group. The strength training group increased their distance walked by 12.4 m (95% CI, -8.42 to 33.3 m) compared to the control group. Neither exercise group improved its short physical performance battery scores. The supervised treadmill group also improved their treadmill walking performance, brachial artery flow-mediated dilation, and QoL. The strength training group improved their treadmill walking QoL and stair climbing ability. Several adverse events occurred related to study participation. One patient had a cardiac arrest during treadmill exercise 4 months after randomization but had non-obstructive disease. Another participant had chest pain during exercise session but was found to not have a flow-limiting lesion and was eventually returned to exercise. Another patient fell during testing and fractured her arm. Another patient developed ankle pain after a session but later returned to complete the trial. The authors conclude, “Based on findings reported in this trial, physicians should recommend supervised treadmill exercise programs for PAD patients, regardless of whether they have classic symptoms of intermittent claudication.”
SET with formal behavioral motivation
Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomized trial. Eur J Vasc Endovasc Surg 2004: 27, 17-23. DOI: 10.1016/j.ejvs.2003.09.012
This randomized trial was designed to evaluate whether supervised exercise is more effective than only the provision of verbal and written advice to exercise. Fifty-nine patients from a regional vascular center were randomized to either exercise advice alone (n = 30) or exercise advice combined with a once a week 45 minute supervised exercise class (n = 29). The mean age was 68 years and 73% male. Assessments included SF-36, Charing Cross Symptom Specific Claudication Questionnaire (CCCQ), and absolute claudication distance. Patient recruitment, inclusion and exclusion criteria are reported. Both patient groups received verbal and written exercise advice of walking at least three times a week to near maximal pain for at least half an hour per session and recommended leg exercises for 6 months. The supervised group had in addition an exercise program conducted under physiotherapy and medical supervision and consisted of walking, leg strengthening, and motivational talks. Patients were assessed at baseline and then at 3, 6, 9, and 12 months. At 6 month follow-up 56 patients were assessed and 55 patients at 12 months with no deaths reportable from vascular events. The CCCQ was improved in the supervised group as compared to the control group, but was statistically significant as compared to baseline only at 9 months. The SF-36 showed a significant difference between the groups in the domain of physical functioning. At all four follow-up assessments median treadmill distance was statistically better in the supervised group as compared to the advice only group. Self-reported compliance showed a significant correlation between drop in CCCQ score, with more than twice as many people in the supervised group reporting compliance. The authors conclude “A weekly, supervised exercise and motivation class for a 6-month period provides a significant improvement in patients’ symptoms, quality of life, and distance walked compared with advice alone and this improvement continues after attendance at class has ceased.”
SET with daily accelerometer feedback
Nicolai SPA, Teijink JAW, Prins MH on behalf of the Exercise Therapy in Peripheral Arterial Disease (EXITPAD) study group. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. J Vasc Surg 2010;52:348-55. DOI: 10.1016/j.jvs.2010.02.022
The EXITPAD randomized trial, funded by The Netherlands Organization for Health Research and Development, compared the effectiveness of exercise advise (the walking advice (WA) group, n=102), SET (n=109), or SET with daily accelerometer feedback (n=93) for patients with IC. Age mean ranged from 66.9 years (SD 8.6) in the WA group, 66.1 years (SD 9.0) in the SET group, and 65.6 years (10.5) in the SET with feedback group. Patients were recruited from eleven outpatient vascular surgery clinics. The WA group received verbal walking advice, a brochure, and instructions to complete 3 exercise sessions per day to maximum pain level. SET was provided by physical therapists and received advice the same as the WA group but in addition received supervised sessions 2 to 3 times weekly initially, then tailored to individual need of the patient during the treatment year. Patients in the SET with accelerometer group received this device in addition to SET. The primary outcome was ACD. Missing values were imputed based on a multivariate linear regression analysis. In the WA group, 15 patients were lost to follow-up and 3 died. In the SET group, 26 discontinued the program with 12 lost to follow-up, 4 died, and the remaining 11 stopped mainly due to lack of motivation. In the SET with feedback, 30% reported nonuse of the accelerometer. Fourteen in this group were lost to follow-up and 13 discontinued the program. Nine patients in the WA group and 13 from the two SET groups together had an intervention which was not statistically different. Data on WIQ and SF-36 was also collected. Results are in Table II and Table III below.

Page 352. Table II. Walking distances in meters (interquartile range). Nicolai SPA, Teijink JAW, Prins MH on behalf of the Exercise Therapy in Peripheral Arterial Disease (EXITPAD) study group. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. J Vasc Surg 2010;52:348-55.
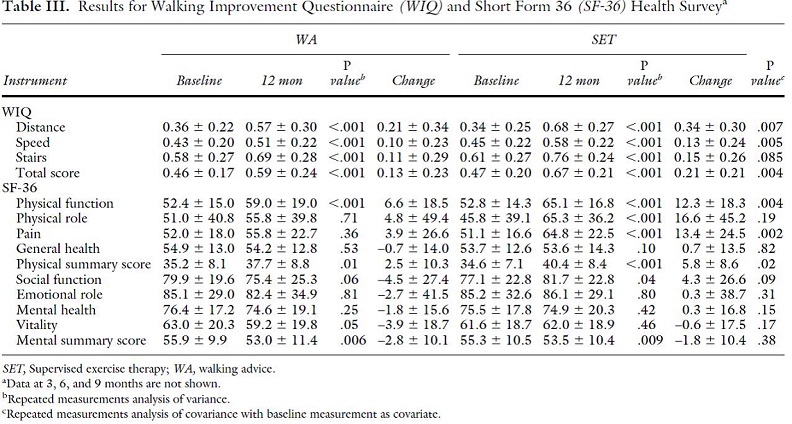
Page 353. Table III. Results for Walking Improvement Questionnaire (WIQ) and Short Form 36 (SF-36) Health Survey. Nicolai SPA, Teijink JAW, Prins MH on behalf of the Exercise Therapy in Peripheral Arterial Disease (EXITPAD) study group. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. J Vasc Surg 2010;52:348-55.
The authors concluded “SET programs should be made available for all patients with intermittent claudication.”
Combination Therapy
Fakhry F, Spronk S, van der Laan L, Wever JJ, Teijink JA, Hoffmann WH, Smits TM, Van Brussel JP, Stultiens GN, Derom A, den Hoed PT, Ho GH, van Dijik LC, Verhofstad N, Orsini M, van Petersen A, Woltman K, Hulst I, van Sambeek MR, Rizopoulos D, Rouwet EV, Hunink MG. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: A randomized clinical trial. JAMA 2015 Nov;314(18):1936-44. DOI: 10.1001/jama.2015.14851
The Endovascular Revascularization and Supervised Exercise (ERASE) trial sought to assess the effectiveness of ER plus SET compared to SET. The trial was sponsored by the Netherlands Organization for Health Research and Development. Participants had to have one or more vascular stenosis at the aortoiliac level, the femoropopliteal level, or both, by noninvasive vascular imaging. Two hundred and twelve patients were randomized to either ER plus SET versus SET only. Mean age was 65 years (SD 10) with 62% men. Fifty-three percent of patients had predominant aortoiliac disease and 47% had predominant femoropopliteal disease. SET was provided by physiotherapists and the program consisted of treadmill walking to near-maximum claudication pain, with a frequency of 2 to 3 sessions per week during the first 3 months, then to one session per week between months 3 and 6 months, later reduced 1 session per 4 weeks. Revascularizations included balloon angioplasty and stenting. No major complications were recorded though 4 patients technically failed the endovascular intervention. The primary endpoint was MWD at 12 months with endpoints measured at 1, 6 and 12 months. Secondary endpoints included PFWD, VascuQoL, and SF-36. MWD improved significantly in both groups though patients in the combination therapy group had statistically greater improvement in MWD with a mean difference at 6 months of 409 m (99% CI 183 – 636 m) and at 12 months of 282 m (99% CI 60 – 505m). Combination therapy had also statistically greater PFWD and disease specific QoL though these measures improved significantly for both groups. For the SF-36 measured domains, only physical functioning was significantly greater at 12 months in the combination therapy group. At 12 months 23 patients (32%) in the combination therapy group had significant restenosis but only 4 underwent a second procedure due to deterioration of claudication. “This suggests that the addition of a supervised exercise program may prevent deterioration despite restenosis or progression of atherosclerotic lesions.” The authors concluded “Among patients with intermittent claudication after 1 year of follow-up, a combination therapy of endovascular revascularization followed by supervised exercise resulted in significantly greater improvement in walking distances and health-related quality-of-life scores compared with supervised exercise only.”
Mazari FAK, Gulati S, Rahman MNA, Lee HLD, Mehta TA, McCollum PH, Chetter IC. Early Outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg 2010; 24:69-79.
This randomized controlled trial compared angioplasty, a supervised exercise program (SEP), and angioplasty plus SEP. One hundred seventy eight patients (108 men, with median age of 70 years) with IC who had unilateral femoropopliteal lesions amenable to angioplasty were randomized over a six-year period. Patients were recruited from a vascular outpatient clinic. All patients were prescribed antiplatelet therapy, received smoking cessation advice, and risk factor modification. All patients also received an advice leaflet on exercise advice. The SEP consisted of 3 session per week for 12 weeks with exercise stations and walking. Sessions were supervised by physiotherapists or physicians. Ankle pressures, treadmill walking distances, SF-36, and VascuQoL data at one and three months were analyzed. Twenty one patients withdrew during the study (SEP group = 8, PTA group = 3, PTA + SEP group = 10). No complications were reported in any group. The authors report that all groups had significant improvements in clinical and QoL measures. Results are in table V below.
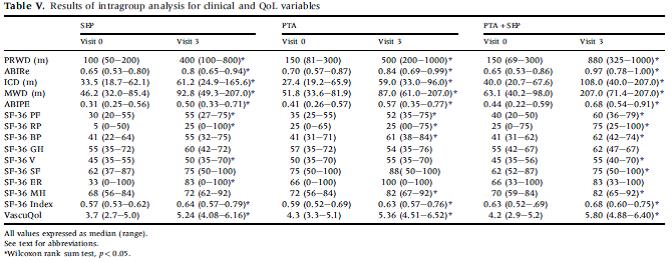
Page 75. Table V. Results of intragroup analysis for clinical and QoL variables. Mazari FAK, Gulati S, Rahman MNA, Lee HLD, Mehta TA, McCollum PH, Chetter IC. Early Outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg 2010; 24:69-79.
The authors conclude “SEP should be the primary treatment for the patients with claudication and PTA should be supplemented by an SEP.”
Prospective Observational Trials without Controls
Community physical activity in older adults with SET
Gardner AW, Katzel LI, Sorkin JD, Killewich LA, Ryan A, Flinn WR, Goldberg AP. Improved functional outcomes following exercise rehabilitation in patients with intermittent claudication. Journal of Gerontology 2000 Oct; 55(10):M570-M577.
Eighty six patients with IC were recruited from a vascular clinic at a Veterans Affairs Health Care System for this study to identify predictors of improved claudication distances following an exercise program and to determine if improved claudication distances translated into increased free-living daily physical activity in the community setting. Patients were characterized on treadmill claudication distances, walking economy (use of oxygen during exercise), peripheral circulation (plethysmography), cardiopulmonary function, self-perceived ambulatory function (WIQ), Minnesota Leisure Time Physical Activity questionnaire), body composition (dual energy x-ray absorptiometry), baseline comorbidities, and free-living daily physical activity (accelerometer on patient’s belt) before and after a 6-month treadmill exercise program. Eighteen patients were noncompliant and 5 did not finish due to new acute medical problems, with 63 patients (mean age 68 ± 1 year; 90% male; 54% African American) successfully completing the study. Cardiovascular risk factors were highly prevalent in this population. The program consisted of supervised, intermittent treadmill walking to near maximal claudication followed by rest, repeated in cycles until the prescribed number of minutes was accomplished. Distance to onset of claudication pain increased 115% (178 m ± 22 m to 383 m ± 34 m) and distance to maximal claudication pain increased by 65% (389 m ± 29 m to 641 m ± 34 m). Peak oxygen uptake increased and the economy of walking at a constant work rate improved. Free-living daily physical activity increased 31% and was associated with increased in treadmill distances to claudication onset and to maximal pain. Self-reported QoL improved as measured by WIQ. The authors note that the increase in distance to onset of claudication pain was related to the baseline age of the patients, however this could be due to lower baseline capacity with age and may better be expressed by relative changes as opposed to absolute.
SET with atherogenic risk factor modification
Franz RW, Garwick T, Haldeman K. Initial results of a 12-week, institution-based, supervised exercise rehabilitation program for the management of peripheral arterial disease. Vascular 2010 Dec; 18(6):325-335. DOI: 10.2310/6670.2010.00053
This study enrolled 122 patients with a diagnosis of PAD or IC in a structured SEP. Twenty one patients elected a self-directed program and were not included in the analysis. Mean age of participants was 64.7 Years (range 37 to 85), with 56.4% males, 43.6% females, 50.5% Caucasians, and 41.6% African-American patients. Supervised exercise sessions in 1 hour intervals were scheduled one to three times a week for 12 weeks. All patients received education regarding disease, athrogenic risk factor modification, lifestyle modification (including smoking cessation), and medication information. Over 60% of patients had a prior intervention. Pre and post program measurements of function, QoL, lipid profiles, weight, blood pressure, and ABI were compared. Thirty (29.7%) patients failed to complete 12 sessions due to a variety of reasons. Statistically significant changes included triglyceride levels, both function tests (12 minute walking test and graded progressive maximal treadmill test), four of five Walking Impairment Questionnaire measurements (walking impairment part A and B, walking distance, walking speed, stair climbing ability), and the Intermittent Claudication Questionnaire score. The authors concluded this supervised program improved the cardiovascular profiles, ambulatory function, and QoL of PAD patients completing the program.
Place of service
Bendermacher BL, Willigendael EM, Nicolai SP, Kruidenier LM, Welton RJ, Hendriks E, Prins MH, Teijink JAW, de Bie RA. Supervised exercise therapy for intermittent claudication in a community-based setting is as effective as clinic-based. J Vasc Surg 2007;45:1192-6. DOI: 10.1016/j.jvs.2007.01.059
This study based in the Netherlands was done to examine the effect of supervised exercise therapy in community based practices that provided physical therapy. Ninety-three patients with IC that met ABI criteria and had no previous peripheral vascular intervention were referred from a vascular outpatient clinic. The mean age is 64.0 ±± 10.4 years with 62.5% men. Thirty seven patients dropped out during the 6 month study time for various reasons including concomitant diseases, interventions, and lack of motivation to continue the program so 56 patients were analyzed. A progressive treadmill test at baseline and at 1, 3, and 6 months of follow-up measured ICD and ACD. Treadmill training was alternated with cardiovascular training and strength training. Patients received two to three session a week of approximately 30 minutes each and later once every 2 weeks. Patients were encouraged to walk to near-maximal pain. ICD improved from baseline (mean of 395 m, with range of 55 to 1600 m) to 3 months (mean of 840 m, with range of 180 to 2260 m) with continued improvement at 6 months (mean of 1005 m, with range of 210 to 3810 m). ACD improved from baseline (mean of 563 m, range 60 to 1700 m) to 3 months (mean of 1154 m, range 290 to 3740 m) with continued improvement at 6 months (mean of 1312 m, range 270 to 3980 m). The authors concluded that supervised exercise therapy at these community based physiotherapy practices provides conservative treatment for patients with IC.
Retrospective Observational Trials
Extended follow-up
Keo HH, Grob E, Guggisberg F, Widmer J, Baumgartner I, Schmid UP, Kalka C, Saner H. Long-term effects of supervised exercise training on walking capacity and quality of life in patients with intermittent claudication. VASA 2008;37(3):250-256. DOI; 10.1024/0301-1526.373.250
Sixty-seven patients with IC who completed a supervised 12-week exercise program at least 6 months before study enrollment were asked to participate in follow-up evaluation. Forty patients agreed to participate, however 2 had angina and 2 could not walk on the treadmill. Of the 36 active participants, the median age was 70 years (range of 46 to 86) with 47.2% males. Comorbidities and concomitant risk factors included cerebrovascular disease, coronary heart disease, obesity, smoking, hypertension, high cholesterol and diabetes. SET consisted of exercise training one hour twice a week between week one to six and one hour per week between weeks seven to twelve. Individual exercise intensity was to exercise as strong and long as possible until near-maximal claudication pain was reached. In addition patients received advice about nutrition, atherosclerotic risk factor reduction, cardiovascular disease education and instruction in home exercise. In these volunteers, PWD and MWD improved compared to baseline as measured after the program (PWD 114 ± 100 vs. 235 ± 248, MWD 297 ± 273 vs. 474 ± 359). When further follow-up was measured (39 ± 20 months) there was continued improvement as compared to baseline (PWD of 197 ± 254 and MWD of 390 ± 324) with non-smokers noted to have better sustained improvement. The authors conclude that improvement in walking capacity is sustained after completion of SET with best results in patients who quit or were non-smokers. They also noted that walking capacity correlated with functional status of QoL.
Sakamoto S, Yokoyama N, Tamori Y, Akutsu K, Hashimoto H, Takeshita S. Patients with peripheral artery disease who complete 12-week supervised exercise training program show reduced cardiovascular mortality and morbidity. Circ J 2009;73(1):167-173.
A study of 118 patients with a clinical diagnosis of IC were enrolled in this retrospective study whose primary endpoint was cardiovascular death. The exercise program consisted of supervised treadmill walking 3 days per week for 12 weeks. Patients were instructed to walk to the point of severe pain and then rest, a cycle that was repeated several times during the hour. The exercise program also included weekly discussion addressing atherosclerotic risk factors and smoking. All patients were encouraged to perform home exercise. Patients were eligible for enrollment in the study after 6 months from the end of exercise training. Clinical outcomes were then assessed from hospital records or telephone questionnaires. Mean follow-up was 5.7 ± 3.9 years (range 0.2 – 13.4). Mean age was 69 years (range 40 to 86). Among the 118 participants, 54.2% (64) completed the 12 week program, with the main reason of non-completion being loss of motivation. No cardiovascular events occurred during the program. MWD in those who completed the program improved from 475.0 m ± 369.0 m to 987.5 m ± 615.5 m. A total of 16 cardiovascular deaths and 51 cardiovascular events occurred among the total 118 participants, with the estimated cardiovascular death-free rate in those who completed the program as compared to those who did not: 98.3% vs 91.5% at 3 years, 95.9% vs 83.4% at 5 years, and 79.9% vs 58.4% at 10 years. Cardiovascular event-free rates of those who completed the program vs those who didn’t: 90.2% vs 85.8% at 3 years; 85.7% vs. 63.7% at 5 years, and 45.7% vs. 38.0% at 10 years. The authors concluded that the study demonstrated that undergoing a 12-week SE training program reduced overall cardiovascular mortality by 52% and morbidity by 30%.
4. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
A MEDCAC meeting was convened to discuss the treatments for lower extremity PAD on July 22, 2015. MEDCAC presentations included evidence in support of Medicare coverage for supervised exercise therapy. Individual MEDCAC panelists voiced support for SET with confidence for inpatient benefit to at least to 18 months. The full transcript and MEDCAC voting results can be accessed here: https://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=70.
In follow-up, the American College of Radiology (ACR), the Society for Cardiovascular Angiography and Interventions (SCAI), the Society of Interventional Radiology (SIR), the Society for Vascular Medicine (SVM), and Vascular InterVentional Advances (VIVA) published an editorial in response to the MEDCAC strongly recommending coverage of SET programs for Medicare patients with IC based on the preponderance of clinical evidence (Shishehbor et al. 2016).
5. Evidence-Based Guidelines
American College of Cardiology (ACC)/American Heart Association (AHA) Practice Guideline
Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S,Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral ArteryDisease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016 Nov 8. pii: S0735-1097(16)36902-9. doi: 10.1016/j.jacc.2016.11.007.
Recommendations for supervised exercise:
- In patients with claudication, a supervised exercise program is recommended to improve functional status and QoL and to reduce leg symptoms. (COR I) (LOE A)
- A supervised exercise program should be discussed as a treatment option for claudication before possible revascularization. (COR I) (LOE B-R)
Supervised exercise program definitions (COR I) (LOE A)
- Program takes place in a hospital or outpatient facility
- Program uses intermittent walking exercise as the treatment modality
- Program can be standalone or within a cardiac rehabilitation program
- Program is directly supervised by qualified healthcare provider(s)
- Training is performed for a minimum of 30-45 minute/session; sessions are performed at least 3 times/week for a minimum of 12 weeks
- Training involves intermittent bouts of walking to moderate-to-maximum claudication, alternating with periods of rest
- Warm-up and cool-down periods precede and follow each session of walking
Class (strength) of recommendation (COR)
COR I is strongly recommended where benefit >>> risk
Level (quality) of evidence (LOE)
LOE A:
High-quality evidence from more than 1 RCT
Meta-analyses of high-quality RCTs
One or more RCTs corroborated by high-quality registry studies
LOE B-R:
Moderate-quality evidence from 1 or more RCTs
Meta-analyses of moderate-quality RCTs
Mirroring the guideline, the AHA has included provision of supervised exercise as a key performance measure for quality-focused PAD patient care. (Olin et al. 2010)
Society for Vascular Surgery (SVS) Practice Guideline
Society for Vascular Surgery Lower Extremity Guidelines Writing Group, Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, Moneta GL, Murad MH, Powell RJ, Reed AB, Schanzer A, Sidawy AN; Society for Vascular Surgery. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015 Mar;61(3 Suppl):2S-41S. doi: 10.1016/j.jvs.2014.12.009.
Recommendations: Exercise therapy
“We recommend as first-line therapy a supervised exercise program consisting of walking a minimum of three times per week (30-60 min/session) for at least 12 weeks to all suitable patients with IC.” Grade 1 level of evidence A
“We recommend home-based exercise, with a goal of at least 30 minutes of walking three to five times per week when a supervised exercise program is unavailable or for long-term benefit after a supervised exercise program is completed.” Grade 1 Level of evidence B
“In patients who have undergone revascularization therapy for IC, we recommend exercise (either supervised or home based) for adjunctive functional benefits.” Grade 1 Level of evidence B
The Grades of Recommendation Assessment, Development and Evaluation (GRADE) framework was used to determine the strength of recommendation and the quality of evidence. The quality of evidence is rated as high (A), moderate (B), or low (C). The rating is based on the risk of bias, precision, directness, consistency, and the size of the effect. The strength of recommendation is graded based on the quality of evidence, balance between benefits and harms, patients’ values, preferences, and clinical context. Recommendations are graded as strong (1) or weak/conditional (2).
National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease: diagnosis and management. NICE clinical guideline CG147. 2012. https://www.nice.org.uk/guidance/cg147
NICE clinical guideline for lower limb PAD management of intermittent claudication recommends that a supervised exercise program be offered to all people with intermittent claudication.
6. Professional Society Recommendations / Consensus Statements / Other Expert Opinion
International Consensus Document
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery. 200; 45(suppl S): S5-S67. PMID: 17223489
Clinical experts from health professional organizations in North America, Europe, Australia, South Africa, and Japan developed a Trans-Atlantic Inter-Society Consensus (TASC II) Document for the management of PAD based on a systematic review of 15 RCTs. There recommendations for patients with IC include:
Supervised exercise should be made available as part of the initial treatment for all patients with peripheral arterial disease. Grade A Recommendation.
The most effective programs employ treadmill or track walking that is of sufficient intensity to bring on claudication, followed by rest, over the course of a 30-60 minute session. Exercise sessions are typically conducted three times a week for 3 months. Grade A Recommendation.
Recommendations are rated by AHRQ criteria where Grade A is “Based on the criterion of at least one randomized, controlled clinical trial as part of the body of literature of overall good quality and consistency addressing the specific recommendation.”
Other Expert Opinion
Numerous independent reviews and expert opinions, some of which were mentioned in the NCD request (Agrawal et al. 2015; Bonaca and Creager 2015; Stewart et al. 2002; Mays and Regensteiner 2014; Hamburg and Balady 2011; Mangiafico and Fiore 2009; Olin and Sealove 2010; Olin et al. 2016; Tattersall et al. 2013; Kirk 2013) are published that support SET for IC.
7. Public Comment
Initial Comment Period: 9/15/2016 – 10/15/2016
During the initial 30-day public comment period CMS received 103 comments, all of which supported Medicare coverage for SET for the treatment of symptomatic PAD. Most of the commenters referenced previous research illustrating the success SET, and many had witnessed patient success first-hand. The majority of comments also referred to the safety and cost-effectiveness of SET as treatment for PAD. There were also several comments indicating PAD patients are seeking more invasive options because SET is not currently covered.
The majority of comments were provided by physicians/cardiologists, exercise physiologists, and nurses. There were a total of six comments on behalf of professional societies/organizations with one comment representing eight groups, including the American College of Cardiology (ACC), American College of Radiology (ACR), AHA, Society for Cardiovascular Angiography and Intervention (SCAI), Society of Interventional Radiology (SIR), Society for Vascular Medicine (SVM), Society for Vascular Surgery (SVS), and Vascular Interventional Advances (VIVA) Physicians. Other groups represented included the American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR), and the Cardiovascular Coalition (CVC). Seventeen comments were comprised by medical program directors, supervisors, and specialists.
All comments that were submitted without personal health information may be viewed in their entirety by using the following link https://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=287
Second Comment Period: 3/2/2017 – 4/1/2017
During the 30-day comment period following the release of the proposed decision memorandum, CMS received 79 comments, with one being omitted from publication on the CMS website due to excessive personal health information content, and one commenter did not include a comment in their submission. All but two commenters expressed explicit support for Medicare coverage of SET for symptomatic PAD. Most of the comments strongly supported our proposed decision. Two comments offered suggestions for the proposed decision without directly expressing support for coverage. No commenters were opposed to expanded Medicare coverage for SET for the treatment of PAD, but some commenters suggested changes with respect to the appropriate conditions of coverage.
The majority of the comments were from physicians/surgeons (31), nurses (12), exercise physiologists (9), and other health professionals (9). There were seven comments on behalf of professional societies/organizations with one comment representing eight groups, including the ACC, ACR, AHA, SCAI, SVM, SVS, SIR, and VIVA Physicians. Other groups represented included the American Physical Therapy Association (APTA), Clinical Exercise Physiology Association (CEPA), AACVPR, Minnesota Association of Cardiovascular and Pulmonary Rehab (MNACVPR), Vascular Cures, and AHA (including the American Stroke Association (ASA)). There was one group comment representing a vascular center, and one industry comment. Six commenters did not identify their title and/or affiliation. The two remaining comments were from a student services advisor and a survivor advocate. CMS appreciates all submitted comments.
Comments on Other Published Materials
Five references that were not previously reviewed were cited in public comments. One of these articles described a small study that focused on the benefit of dynamic arm exercise for treating claudication, while another highlighted the effectiveness of treadmill walking compared to strength training for patients with PAD. There was one article describing the features of an ideal exercise program, and one describing the Dutch ClaudicatioNet concept. The final article was a Presidential Address from the New England Society of Vascular Surgery which illustrated the effectiveness of exercise therapy for treating IC. While these articles referenced SET and were generally supportive, they did not meet study inclusion and exclusion criteria to be added to the analysis or were outside the scope of this decision. Nonetheless we appreciate that commenters submitted published articles to assist in our review.
Direct Supervision
Comment: Several commenters disagreed with the requirement that SET be under the “direct supervision” of a physician, suggesting non-physician providers are capable of supervision. Many of these commenters suggested that “general supervision” would be appropriate.
Response: CMS appreciates the comment. After review, we agree that it would be appropriate for qualified non-physician practitioners (identified in 1861(aa)(5)) to also provide direct supervision for SET, as long as they are trained in both basic and advanced life support techniques. The non-physician practitioners are physician assistants and nurse practitioners/clinical nurse specialists. Qualified auxiliary personnel may participate in providing SET.
Role of Physical Therapists
Comment: Two comments were specific to physical therapists and what their role would be during SET sessions since they are not specifically mentioned in the decision.
Response: While physical therapists are not included in the list of non-physician practitioners, they may participate as qualified auxiliary personnel who may be able to deliver the service.
Physician Referral
Comment: Two commenters questioned the need for a “face-to-face” visit with a physician being required for referral to SET, both stating that a referral for SET could come from a physician or non-physician practitioner who is familiar with the patient’s cardiovascular health and PAD. It was also mentioned that a patient may receive the face-to-face visit in hospital (after an admission) but it would be difficult to document, requiring an additional visit for the referral.
Response: We believe that a face-to-face meeting with the physician responsible for PAD treatment to obtain the referral for SET is an important step for the patient to actively engage in informed decision making with the physician familiar with their condition. This physician would have the best overall knowledge of treatment options and knowledge of patient condition. This face-to-face meeting could occur in the hospital but similar to all encounters between a patient and physician, the visit is documented in the medical record. The visit also provides an opportunity to discuss behavioral risk factor reduction including, but not limited to, initiating and maintaining long term, regular physical activity to improve overall health.
Staff Requirements
Comment: Two comments questioned the need for qualified auxiliary personnel to be trained in both basic and advanced life support techniques and in exercise therapy for PAD. Both comments indicated this was inconsistent with the terminology for staff qualifications in 42 CFR 410.49 “Cardiopulmonary training in basic life support or advanced life support.”
Response: CMS agrees and clarifies that the basic and advanced life support requirement applies only to the personnel that meet the direct supervision.
Setting
Comment: Several commenters suggested expanding the place of service beyond the hospital or outpatient hospital setting to also include physician offices. One comment also suggested expanding to other settings, such as skilled nursing facilities.
Response: CMS agrees that SET provided in the physician office setting has been proven to improve outcomes in clinical studies. After further evaluation, the place of service has been modified to hospital outpatient settings and physician offices. While we appreciate the suggestion of expanding to other settings such as skilled nursing facilities, the evidence reviewed does not mention these settings as appropriate for SET. We believe hospital outpatient settings and physician offices are the most appropriate settings for SET for PAD, and is consistent with the settings for cardiac rehabiliation programs.
Contraindications
Comment: Several commenters requested that we remove “amputation” from the list of contraindications for SET, indicating that an amputation would not necessarily prevent someone from being able to exercise, and may help prevent the loss of a second limb. Other commenters suggested that the patients’ attending physician should determine whether there were contraindications that might preclude this service.
Response: CMS agrees that an amputation may not prohibit SET as these patients may have a prosthesis that will enable them to walk. CMS also agrees that the patient’s primary physician may be the best judge of exercise contraindications. We have modified our decision accordingly.
Number of Sessions
Comment: Some commenters suggested changing the requirement of 3 sessions per week (36 sessions over 12 weeks) to a less prescriptive “up to” 36 sessions over 12 weeks, indicating that the number of sessions needed per week may vary by patient and there could be scheduling conflicts and transportation difficulties that prevent attendance 3 times per week.
Response: CMS would like to highlight that the ACC/AHA guidelines for SET recommend at least 3 sessions per week. However, we understand that flexibility is needed from a practical standpoint and is important for individual patients to maximixe benefits. We have modified our decision accordingly.
Smoking Cessation
Comment: One commenter suggested adding smoking cessation services to SET for PAD.
Response: While we agree smoking cessation is an important part of treating PAD, SET for the treatment of symptomatic PAD is limited to sessions of exercise. CMS believes that smoking cessation services or referral for services should be addressed at a patient’s face-to-face referral visit or at regular follow-ups with their treating physician. We note that Medicare already covers counseling to prevent tobacco use as a separate benefit under part B.
Concern about Medicare Copayments
Comment: One commenter was concerned that high Medicare copayments may discourage patients from getting this service.
Response: By expanding Medicare coverage through our NCD, we are providing access to care for this service under the Part B program. As is the case for most services under part B, the statute requires payment of coinsurance or cost-sharing. We do not have the authority to eliminate the coinsurance requirement.
VIII. CMS Analysis
National coverage determinations are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1869(f)(1)(B) of the Act). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member (§1862(a)(1)(A) of the Act).
When making national coverage determinations, we evaluate the evidence related to our analytic questions based on the quality, strength and totality of evidence presented in the reviewed literature. As part of this evaluation, it is important to consider whether the evidence is relevant to the Medicare beneficiary population. In determining the generalizability of the results of the body of evidence to the Medicare population, we consider, at minimum, the age, race and gender of the study participants.
In this analysis, we addressed the question(s) below:
Is the evidence sufficient to conclude that SET improves health outcomes for Medicare beneficiaries with IC due to PAD?
If the answer to the question above is positive, is the available evidence sufficient to identify program characteristics that are more likely to lead to overall benefit from SET?
Disease Background Summary: Atherosclerosis of the lower extremity arteries is called peripheral artery disease (PAD). Coexisting coronary and cerebral atherosclerosis is common as atherosclerosis is a systemic vascular condition. In patients with PAD, 61% have coronary artery disease (CAD) and/or cerebrovascular disease (CBVD) (Criqui 2015). PAD can be asymptomatic, but as it progresses it can cause symptoms of pain and limit daily physical activities. Patients with IC may have progression to critical limb ischemia, where damage may be irreversible and lead to amputation. Increasing sharply with age, PAD affects 5% of those over 55 years and greater than 10% in those in their 60’s and 70’s (Criqui 2015). The primary symptom is lower extremity pain from impaired circulation, called claudication. In IC, the typical pain occurs in the muscles of the calf, thigh or buttock and comes on only after walking and is relieved by resting. IC is in the disease spectrum of asymptomatic PAD and critical limb ischemic. Risk factors for the development of lower limb arterial disease are similar to those for coronary artery disease and include age, high cholesterol, hypertension, diabetes, gender, renal insufficiency and smoking.
Treatments for IC due to PAD may include medications, cardiovascular risk reduction, exercise therapy and endovascular revascularization. This decision focuses on supervised exercise therapy (SET).
Patient Characteristics: In regards to PAD, the patients are more similar than dissimilar. The average age of PAD study participants is generally within the Medicare population and predictable concomitant comorbidities were reported in many of the studies. In general, studies enrolled adequate samples of women. Ethnicity was more variable. Based on the patient characteristics the results of the study population are generalizable to the Medicare population.
Exercise as a Treatment: Clinical effectiveness and safety of exercise therapy has been studied for decades and the evidence base contains a number of systematic reviews. Many of the individual trials are small but have similar endpoints of standardized treadmill outcome metrics and disease specific QoL questionnaires. Inclusion criteria vary somewhat, but in general include Fontaine stage 2 patients Stage II (i.e., mild claudication pain in limb).
Systematic reviews have examined the effectiveness of SET versus usual care, SET versus unsupervised exercise (UE), SET versus other IC therapies and additionally SET safety. The rest of the studies we evaluated concluded positive results when comparing SET to UEA Cochrane review by Lane et al. (2014) analyzed 30 RCTs with 1816 participants with mean ages between 65 and 67 years and concluded that exercise programs clearly improve walking time and distance (measures such as PFWD, PFWT, MWT, MWD) compared to controls or medical therapy, with benefits appearing to be sustained for over two years. In addition, Murphy et al. (2015) reported results of a randomized controlled trial of 111 participants with mean age of 64 years and concluded that supervised exercise provides durable improvement in functional status (peak treadmill walking time) and quality of life (Peripheral Artery Questionnaire) up to 18 months. The largest U.S. study (systematic review by Jones et al. 2013) analyzed all treatments for PAD and results were presented at the MEDCAC. They reported that SET and SET with ER resulted in large improvements in maximal walking distance in comparison with usual care, with moderate strength of evidence.
Systematic reviews have also demonstrated the SET may be conducted with few harms in patients with IC due to PAD. A systematic review by Gommans et al. (2015) analyzed 74 studies with 2876 patients with IC (82,725 patient-hours of SET) with mean age of 64 years and concluded that SET can be safely administered in patients with IC “because an exceedingly low all-cause complication rate was found.” Overall the totality of evidence supports SET as an effective therapy for IC. The evidence basis on SET is substantial and positive for improvements in exercise capacity/functional status. The benefits far exceed the harms.
Supervision, an important consideration for program construction and implementation, has also been evaluated extensively. A Cochrane review by Fokkenrood et al. (2013) analyzed 14 RCTs with 1002 patients with a mean age of 67 years and concluded that supervised exercise therapy provides statistically significant benefits for treadmill walking distance when compared with non-supervised programs, with benefits up to 12 months. In addition, a systematic review by Vemulapalli (2015) analyzed 27 studies (24 RCTs) with 2074 patients and concluded that supervised exercise is more effective than unsupervised exercise at improving maximal walking and claudication distances. The authors noted that improved compliance is one of the potential mechanisms of benefit for supervised exercise. The evidence strongly supports supervision.
Outcomes: The large majority of studies of SET evaluated exercise capacity as assessed by a number of walking measures including 6 minute walk test, ACD, AWD, MWD, MWT, PFWD, PFWT and PWT. We noted that exercise capacity is an intermediate outcome and that exercise capacity alone would not be an appropriate endpoint; however, it is a meaningful outcome for IC due to symptomatic PAD since there is a well-established evidence link to all-cause mortality (Georgiopoulou et al., 2017; Morris et al., 2014; Yazdanyar et al., 2014). This is supported by the observational study by Franz et al. (2010) which showed supervised exercise improved cardiovascular profiles and the retrospective study by Sakamoto et al. (2009) that reported a reduction in overall cardiovascular mortality from supervised exercise training. Since patients have associated cardiovascular diseases like CAD and CHF, evidence that supervised exercise therapy reduces mortality in trials of other cardiovascular diseases is also relevant. For example, HF-ACTION investigators (O’Connor et al., 2009) reported that “after adjustment for highly prognostic predictors of the primary end point, exercise training [36 supervised sessions] was associated with modest significant reductions for both all-cause mortality or hospitalization and cardiovascular mortality or heart failure hospitalization.”
After the analysis of all of the studies, we conclude that exercise in a supervised clinical environment showed positive health benefits that contribute to the finding of improved outcomes. Supervision provides for efficient continuous improvements in workload; offers immediate encouragement and support; accomplishes immediate measurement to support accomplishment; monitors for acute medical issues should they arise in this vulnerable population; provides additional encouragement and feedback for risk factor reduction such as smoking cessation, blood pressure control, and diet.
Duration of Benefits: Studies have shown that benefits of SET for patients with IC due to PAD are durable with findings up to 12 months, (Fokkenrood et al., 2013), 18 months (Murphy et al., 2015), 2 years (Lane et
al., 2014) and 39 months (Keo et al., 2008).
Other Findings: We note that observational data provides additional supportive and informative evidence. We reviewed the following: Gardner (2000) demonstrated improved disease specific QoL scores and increased daily physical activity in addition to improved walking parameters with a 6 month SET program. Franz (2010) concluded that triglyceride levels and disease specific QoL scores improved, as did walking parameters with a 3 times a week, 12 week supervised program. Keo (2008) found that walking capacity correlated with QoL. These studies help to define the duration and parameters of SET.
Program characteristics: SET for IC is structured differently than cardiac rehabilitation, as lower extremity claudication is the limiting factor for walking. A SET program follows a pattern of short periods of walking that bring on moderate intensity symptoms of claudication, alternated with short rest periods, and is based on sequential increases in workload while walking on a treadmill. While various types of exercise have been evaluated through the years, progressive treadmill walking appears to be the best at alleviating claudication symptoms (Regensteiner, 1996). For instance, lower extremity strength training has been hypothesized to increase function in PAD patients as adults with PAD have smaller calf muscle area and poorer leg stretch than those without PAD (McDermott, 2009). However, trials with lower extremity strength training in PAD in comparison to progressive treadmill training have had inferior walking parameter outcomes. One example of a comprehensive therapeutic exercise program for IC was reported by Stewart et al. (2002):

Page 1947. Table 4. Key elements of a therapeutic exercise-training program for rehabilitation for peripheral arterial disease in patients with claudication. Stewart KR, Hiatt WR, Regensteiner JG, Hirsch AT. Medical progress: Exercise training for claudication. NEJM 2002 Dec;347:1941-1951.
In the published studies reviewed, program characteristics were variable. The type of exercise was variable but usually involved walking either treadmill or corridor. SET sessions generally ranged from 2 to 3 times per week for 12-24 weeks. Optimal medical therapy and cardiovascular risk factor reduction were baseline interventions. Given the variability among published studies, professional society recommendations can help establish program parameters.
The ACC/AHA recommends: Supervised exercise program definitions (COR I) (LOE A)
- Program takes place in a hospital or outpatient facility
- Program uses intermittent walking exercise as the treatment modality
- Program can be standalone or within a cardiac rehabilitation program
- Program is directly supervised by qualified healthcare provider(s)
- Training is performed for a minimum of 30-45 minute/session; sessions are performed at least 3 times/week for a minimum of 12 weeks
- Training involves intermittent bouts of walking to moderate-to-maximum claudication, alternating with periods of rest
- Warm-up and cool-down periods precede and follow each session of walking
The SVS recommends “as first-line therapy a supervised exercise program consisting of walking a minimum of three times per week (30-60 min/session) for at least 12 weeks to all suitable patients with IC.” (Grade 1 level of evidence A).
Limitations: Though there is general agreement that SET is beneficial for patients with IC based on clinical evidence, there are some quality issues with the studies. These quality of evidence issues are well articulated by NICE in their 2012 review: Effect sizes tend to be small; individual studies are generally rated moderate to low quality using GRADE criteria; trials differ by types of exercise; improvements could be related to an increase in healthcare provider contact; treadmill walking has a training effect; the data on withdrawals is limited and may not be generalizable; definitions of IC varied; long term benefits are unclear. Exercise capacity may be subject to bias if not objectively and consistently measured. It is important to maintain validity and reliability of measures. Despite these study interpretation limitations, the totality of evidence for SET in patients with symptomatic PAD with IC suggests patient benefit with strong evidence of efficacy and low to non-existent evidence of risk.
Contraindications: Not all patients with PAD will be able to safely undergo exercise training. The AHA recommends in the case of general exercise training, “In particular, patients with established heart disease or other selected chronic medical conditions should undergo a medical evaluation to ensure clinical stability, to provide specific activity recommendation, and to guide a safe progression with exercise training.”(Fletcher 2013) Risk stratification tables are provided in Fletcher et al. 2013. Beyond unstable cardiovascular disease such as critical limb ischemia, unstable angina, decompensated heart failure, uncontrolled cardiac arrhythmias, severe symptomatic valvular heart disease, hemodynamic instability at rest or in response to exercise, individuals may have non-cardiovascular disabilities that preclude safe exercise, such as orthopedic, neurologic, or mental impairment.
Guidelines: SET for patients without co-morbidities precluding the safe use of exercise is supported by guidelines and cardiovascular, vascular, and rehabilitative professional organizations. The provision of SET as initial treatment is also the international standard of care. The 2016 AHA/ACC guideline on the management of lower extremity PAD recommendation for SET in patients with claudication is based on the highest level of evidence, a level 1A recommendation. SET is recommended as an initial therapy for IC as well as with endovascular therapies. The provision of SET is endorsed as a key quality measure by AHA. The SVS also recommended supervised exercise for patients with IC with Grade l, Level of evidence A. Many published independent expert reviews support SET for treatment of IC. CMS held a MEDCAC in July 2015 to discuss PAD treatments. One of the broadly endorsed recommendations resulting from the MEDCAC was coverage of SET. This recommendation was based on the totality of clinical evidence. As a follow-up to the MEDCAC, a consortium of vascular stakeholders (Shishehbor et al., 2016) authored an editorial recommending the coverage of SET based on the preponderance of the evidence presented at the meeting. The National Institute for Health and Clinical Excellence (NICE) conducted an analysis for guideline development and concluded that SET is superior to unsupervised exercise and may have superior outcomes in walking distance in certain circumstances.
Based on the totality of the evidence including public comments, we believe there is sufficient evidence to conclude that SET improves health outcomes for Medicare beneficiaries with IC due to PAD. There is high quality, high strength evidence from several systematic reviews and randomized controlled trials on the benefits of SET for individuals with IC for the treatment of symptomatic PAD. Trials showed that SET decreases mortality, reduces cardiovascular risk factors, increases exercise capacity, and increases quality of life in older adults. While in general exercise capacity alone, which was an endpoint in a number of studies, would not be an appropriate outcome, it is a meaningful outcome for IC due to symptomatic PAD since there is a well-established evidence link to all-cause mortality. The benefits of SET exceed the minimal reported harms since it is a non-invasive supervised treatment. Professional societies such as the ACC, AHA, SVS, NICE and the TASC have also recommended SET for individuals with IC for the treatment of symptomatic PAD.
During the second public comment period, CMS received 79 comments. CMS appreciates the comments and published articles. All but two commenters expressed explicit support for Medicare coverage of SET for symptomatic PAD; the other two comments offered suggestions for the proposed decision without directly expressing support for coverage. No commenters were opposed to expanded coverage. Five references were cited in public comments and evaluated. While these articles were supportive of supervised exercise therapy for symptomatic PAD, they either did not meet study inclusion and exclusion criteria to be added to the analysis or were outside the scope of the analysis.
If the answer to the question above is positive, is the available evidence sufficient to identify program characteristics that are more likely to lead to overall benefit from SET?
A SET program follows a pattern of short periods of walking that bring on moderate intensity symptoms of claudication, alternated with short rest periods, and is based on sequential increases in workload while walking on a treadmill. While various types of exercise have been evaluated through the years, progressive treadmill walking appears to be the best at alleviating claudication symptoms (Regensteiner, 1996).
As discussed earlier, the published guidelines indicate patient benefit from a minimum of 12 weeks of SET. We do not expect that all patients will require, or that physicians will support, more than 12 weeks of SET. We do not want to limit SET coverage as a once in a lifetime benefit since we also understand that some patients may continue to benefit after 12 weeks of SET and want to provide flexibility to allow for additional sessions. We note that some question why SET for IC should be a separate service and not part of cardiac rehabilitation. SET for IC is structured differently than cardiac rehabilitation, as lower extremity claudication is the limiting factor for walking. Similar to our coverage for cardiac rehabilitation, we believe that beneficiaries and the Medicare program will be best served if beneficiaries have access to up to 36 additional sessions over an extended period of time at the discretion of the MACs based upon each individual patient’s specific circumstances. Current professional society guidelines (ACC, AHA, SVS) are available and provide evidence-based recommendations on SET program parameters. Program elements have also been published. Based on the available evidence, program characteristics can be identify to ensure the benefits of SET exceed harms.
Much of the care for PAD patients is not guideline directed (Hirsch 2001; MEDCAC 2015) and even more alarming is that amputation rates in certain regions of the country remain elevated. Coverage of this evidence-based service should serve as a first-step to further adopt and adhere to evidence-based care for all PAD patients in the Medicare population.
Patient-Centered Considerations: From a patient’s standpoint, what are the current limitations of SET in the real world clinical environment? This question can be answered with current data from trials by examining reasons for study dropout. Patients must be motivated. Lack of reimbursement has been a barrier. Transportation can be a barrier. In addition, exercise therapy requires a significant time commitment on the part of the patient and provider. It is important to note that appropriate, adequate physical activity should be continued regularly over time to sustain the benefits achieved from SET. Recidivism is a concern. A long term commitment is required. Further
research is needed to improve motivational factors, an issue that is common for behavioral therapies.
Health Disparities
While studies examining SET have observed similar results of success, no study has examined its effectiveness among different races/ethnicities. African Americans are particularly vulnerable to PAD. Using the ABI criteria of less than 0.90 for the diagnosis of PAD, the prevalence is 2.5 times greater among African Americans than Caucasians, with the prevalence of PAD being higher in African Americans than in Hispanics and Caucasians combined (Rucker-Whitaker et al., 2004). In addition, African Americans suffering from PAD were approximately 50% less likely than Caucasians to have revascularization treatment.
In a study by Rucker-Whitaker et al. (2004), African Americans with PAD had a significantly lower ABI, and a higher prevalence of leg pain (both with and without exertion) than Caucasians with PAD. It was also found that African Americans with PAD had significantly shorter 6-minute walk distances, and significantly slower 4-meter walking velocities compared to Caucasians with PAD. Given the significantly higher prevalence of PAD and claudication, coupled with the decreased likelihood to seek treatment among African Americans, we encourage patients to be vigilant about IC symptoms and to seek treatment early and practitioners to provide appropriate evidence-based treatments. This highlights the need for future research to emphasize PAD treatment effectiveness for African American patients.
We reviewed a single study (Gommans (2015)) that examined gender differences. Gommans concluded women benefit less during the first 3 months of SET and have lower AWDs after 12 months in comparison with men, and hypothesized that gender based treatment strategies may be required to optimize outcomes in women.
Summary
The overarching concept to maintain or improve health is extremely important: participation in regular physical activity is associated with a reduction in the risk of cardiovascular events, a greater life expectancy, and an improvement in exercise capacity and QoL. For those in the Medicare population who are limited in walking from the pain of claudication, significant evidence exists to support coverage of SET. In addition, these patients should have additional risk factor modification therapies and disease education activities. The evidence base for SET is at least equivalent to already covered therapies for IC. The evidence is sufficient to conclude that supervised exercise therapy improves health outcomes for Medicare beneficiaries with intermittent claudication due to PAD. We encourage all providers to adopt and adhere to evidence based care for patients with PAD. To sustain benefits over time, a long term commitment to appropriate, adequate physical activity by providers and patients is needed.
IX. Conclusion
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to cover supervised exercise therapy (SET) for beneficiaries with intermittent claudication (IC) for the treatment of symptomatic peripheral artery disease (PAD). Up to 36 sessions over a 12 week period are covered if all of the following components of a SET program are met:
The SET program must:
- consist of sessions lasting 30-60 minutes comprising a therapeutic exercise-training program for PAD in patients with claudication;
- be conducted in a hospital outpatient setting, or a physician’s office;
- be delivered by qualified auxiliary personnel necessary to ensure benefits exceed harms, and who are trained in exercise therapy for PAD; and
- be under the direct supervision of a physician (as defined in 1861(r)(1)), physician assistant, or nurse practitioner/clinical nurse specialist (as identified in 1861(aa)(5)) who must be trained in both basic and advanced life support techniques.
Beneficiaries must have a face-to-face visit with the physician responsible for PAD treatment to obtain the referral for SET. At this visit, the beneficiary must receive information regarding cardiovascular disease and PAD risk factor reduction, which could include education, counseling, behavioral interventions, and outcome assessments.
- Medicare Administrative Contractors (MACs) have the discretion to cover SET beyond 36 sessions over 12 weeks and may cover an additional 36 sessions over an extended period of time. A second referral is required for these additional sessions.
- SET is non-covered for beneficiaries with absolute contraindications to exercise as determined by their primary physician.
See Appendix B for the NCD manual language.
APPENDIX A
General Methodological Principles of Study Design
(Section VI of the Decision Memorandum)
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to that group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is to the extent that differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well-designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of that have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
Randomized controlled trials
Non-randomized controlled trials
Prospective cohort studies
Retrospective case control studies
Cross-sectional studies
Surveillance studies (e. g. , using registries or surveys)
Consecutive case series
Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in that confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to that the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
APPENDIX B
Medicare National Coverage Determinations Manual
Draft
This information is representative of Medicare's national coverage determination (NCD) for implementation purposes only. The information is subject to formal revisions and formatting changes prior to the release of the final NCD contractor instructions and publication in the Medicare National Coverage Determinations Manual.
Table of Contents
(Rev.)
[XXX.X]
A. General
Research has shown SET to be an effective, minimally invasive method to alleviate the most common symptom associated with PAD (IC). SET has been shown to be significantly more effective than unsupervised exercise, and could prevent the progression of PAD and lower the risk of cardiovascular events that are prevalent in these patients. SET has also been shown to perform at least as well as more invasive revascularization treatment, which is covered by Medicare.
B. Nationally Covered Indications
Effective for services performed on or after May 25, 2017 CMS has determined that the evidence is sufficient to cover supervised exercise therapy (SET) for beneficiaries with intermittent claudication (IC) for the treatment of symptomatic peripheral artery disease (PAD). Up to 36 sessions over a 12 week period are covered if all of the following components of a SET program are met:
The SET program must:
- consist of sessions lasting 30-60 minutes comprising a therapeutic exercise-training program for PAD in patients with claudication;
- be conducted in a hospital outpatient setting, or a physician’s office;
- be delivered by qualified auxiliary personnel necessary to ensure benefits exceed harms, and who are trained in exercise therapy for PAD; and
- be under the direct supervision of a physician (as defined in 1861(r)(1)), physician assistant, or nurse practitioner/clinical nurse specialist (as identified in 1861(aa)(5)) who must be trained in both basic and advanced life support techniques.
Beneficiaries must have a face-to-face visit with the physician responsible for PAD treatment to obtain the referral for SET. At this visit, the beneficiary must receive information regarding cardiovascular disease and PAD risk factor reduction, which could include education, counseling, behavioral interventions, and outcome assessments.
Medicare Administrative Contractors (MACs) have the discretion to cover SET beyond 36 sessions over 12 weeks and may cover an additional 36 sessions over an extended period of time. A second referral is required for these additional sessions.
C. Nationally Non-Covered Indications
SET is non-covered for beneficiaries with absolute contraindications to exercise as determined by their primary physician.
D. Other
NA


