TO: Administrative File: (CAG-00436N)
FROM: Tamara Syrek Jensen, JD
Acting Director
Coverage and Analysis Group
Lori Ashby
Acting Director
Division of Medical and Surgical Services
Lori Paserchia, MD
Lead Medical Officer
Deirdre O'Connor
Lead Analyst
Division of Medical and Surgical Services
SUBJECT: Decision Memorandum for Screening for Hepatitis C Virus (HCV) in Adults
DATE: June 2, 2014
I. Decision
The Centers for Medicare & Medicaid Services (CMS) has determined the following:
The evidence is adequate to conclude that screening for Hepatitis C Virus (HCV), consistent with the grade B recommendations by the U.S. Preventive Services Task Force (USPSTF), is reasonable and necessary for the prevention or early detection of an illness or disability and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B, as described below.
Therefore, CMS will cover screening for HCV with the appropriate U.S. Food and Drug Administration (FDA) approved/cleared laboratory tests, used consistent with FDA approved labeling and in compliance with the Clinical Laboratory Improvement Act (CLIA) regulations, when ordered by the beneficiary's primary care physician or practitioner within the context of a primary care setting, and performed by an eligible Medicare provider for these services, for beneficiaries who meet either of the following conditions.
- A screening test is covered for adults at high risk for Hepatitis C Virus infection. “High risk” is defined as persons with a current or past history of illicit injection drug use; and persons who have a history of receiving a blood transfusion prior to 1992. Repeat screening for high risk persons is covered annually only for persons who have had continued illicit injection drug use since the prior negative screening test.
- A single screening test is covered for adults who do not meet the high risk as defined above, but who were born from 1945 through 1965.
The determination of “high risk for HCV” is identified by the primary care physician or practitioner who assesses the patient's history, which is part of any complete medical history, typically part of an annual wellness visit and considered in the development of a comprehensive prevention plan. The medical record should be a reflection of the service provided.
For the purposes of this national coverage determination (NCD), a primary care setting is defined by the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community. Emergency departments, inpatient hospital settings, ambulatory surgical centers, independent diagnostic testing facilities, skilled nursing facilities, inpatient rehabilitation facilities, clinics providing a limited focus of health care services, and hospice are examples of settings not considered primary care settings under this definition.
For the purposes of this NCD, a “primary care physician” and “primary care practitioner” will be defined consistent with existing sections of the Social Security Act (§1833(u)(6), §1833(x)(2)(A)(i)(I) and §1833(x)(2)(A)(i)(II)).
§1833(u)
(6) Physician Defined.—For purposes of this paragraph, the term “physician” means a physician described in section 1861(r)(1) and the term “primary care physician” means a physician who is identified in the available data as a general practitioner, family practice practitioner, general internist, or obstetrician or gynecologist.
§1833(x)(2)(A)(i)
(I) is a physician (as described in section 1861(r)(1)) who has a primary specialty designation of family medicine, internal medicine, geriatric medicine, or pediatric medicine; or
(II) is a nurse practitioner, clinical nurse specialist, or physician assistant (as those terms are defined in section 1861(aa)(5));
II. Background
The following acronyms are used throughout this document. For the readers convenience they are listed here in alphabetical order.
AAFP – American Academy of Family Physicians
AASLD – American Association for the Study for Liver
Diseases
ACG – American College of Gastroenterology
AHRQ – Agency for Health Research and Quality
CDC – Centers for Disease Control and Prevention
CLIA – Clinical Laboratory Improvement Act
DAA – direct-acting antiviral agent
ESRD – end stage renal disease
FDA – U.S. Food and Drug Administration
HAV – Hepatitis A virus
HBV – Hepatitis B virus
HCV – Hepatitis C virus
HCC – Hepatocellular carcinoma
HIV – Human immunodeficiency virus
IDSA – Infectious Disease Society of America
IOM – Institute of Medicine
MMWR – Morbidity and Mortality Weekly Report
NANBH – non-A, non-B viral Hepatitis
NAT – nucleic acid test
NCA – National Coverage Analysis
NCD – National Coverage Decision
PR – pegylated interferon plus ribavirin
QALY – quality adjusted life years
RR – relative risk
SAE – serious adverse events
SVR – sustained virologic response
USPSTF – United States Preventive Services Task Force
Based upon publication of updated HCV screening guidelines by the USPSTF, CMS initiated this national coverage analysis (NCA) to evaluate the existing evidence on HCV screenings for adults. The scope of this NCA includes a review of the existing evidence and a determination if the body of evidence is sufficient for Medicare coverage of screening for HCV in adults, which is recommended with a grade B by the USPSTF. “The USPSTF recommends screening for hepatitis C virus (HCV) infection in persons at high risk for infection. The USPSTF also recommends offering one-time screening for HCV infection to adults born between 1945 and 1965.” (http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm)
For the purposes of this NCA, we are furnishing information on viral hepatitis as an inflammation of the liver caused by hepatitis A, B, C, D, or E viruses. We are not discussing hepatitis arising from other viral agents, e.g. Epstein-Barr virus (EBV) or cytomegalovirus (CMV). The identification of non-A, non-B viral hepatitis (NANBH) followed the 1975 advent of serological testing for hepatitis A (HAV) and B (HBV) and the realization that HAV and HBV did not account for most cases of transfusion-associated hepatitis. (Houghton 2009) By 1988, the hepatitis C virus (HCV) had been identified and was shown to be the principal cause of parenterally transmitted NANBH. (Houghton 2009) While the basic structure is common to all HCVs, there are at least six different genotypes of the virus. (Rosen 2011) Genotype 1 is the most common in the U.S. (Chou, Hartung 2013)
HCV is an infection that attacks the liver and leads to inflammation. The infection is often asymptomatic and can go undiagnosed for decades. It is difficult for the human immune system to eliminate the HCV and it is a major cause of chronic liver disease. The presence of HCV in the liver initiates a response from the immune system which in turn causes inflammation. Inflammation over long periods of time (usually decades) can cause scarring, called cirrhosis. A cirrhotic liver fails to perform the normal functions of the liver, which leads to liver failure. Cirrhotic livers are more prone to become cancerous and liver failure leads to serious complications, even death. HCV is reported to be the leading cause of chronic hepatitis, cirrhosis and liver cancer and a primary indication for liver transplant in the Western World. (Rosen 2011) “The morbidity and mortality associated with chronic HCV are mainly attributable to its progression toward cirrhosis and hepatocellular carcinoma.” (Rauch 2010)
About 80% of people exposed to HCV develop chronic infection and of these three to 11% will develop liver cirrhosis within 20 years. (Nelson 2011) The natural history and chronicity rates for people infected with the HCV vary. The possible outcomes of HCV are diagramed below.
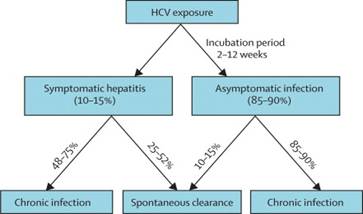
(Figure 3: Outcome of HCV infection, Maheshwari et al. 2011)
Risk
HCV is a bloodborne infection and risks for transmission are primarily associated with exposures to contaminated blood or blood products via transfusions, shared needles and reused medical supplies. “Sexual and mother-to-child transmission is much less likely than for HIV. In developed nations, most new infections occur in injection-drug users.” (Gravitz 2011) By 1992 serologic assays for the testing of the blood supply had been developed. This allowed for effective screening of blood donors.
The USPSTF identified the most important risk factors for HCV infection as past or current injection drug use or the receipt of a blood transfusion before 1992. (USPSTF Screening for Hepatitis C Virus in Adults 2013) The Centers for Disease Control and Prevention (CDC) noted that in 1998 the highest prevalence of HCV was identified among persons with substantial or repeated direct percutaneous exposures, such as people who inject drugs, those who receive blood from infected donors, and persons with hemophilia. (CDC MMWR Vol. 61/No. 4 August 17, 2012)
In the United States, for the population already infected with HCV, veterans and baby boomers (those born between 1946 and 1964) are most at risk for becoming symptomatic. Veterans have an infection rate at least three times that of the general population and baby boomers, who make up about 30% of the U.S. population, account for two-thirds of the people with HCV in the US. (Gravitz 2011)
Epidemiology
Infection with HCV affects more than 180 million people globally. In 2010, the CDC estimated that 2.7 to 3.9 million persons in the United States were living with HCV. (CDC MMWR/Vol.61/No. 4 August 17, 2012)
A safer blood supply along with safer injection practices among intravenous drug users contributed to a decline in the number of reported cases of HCV from 1999 – 2008. (CDC MMWR/Vol61/No. 4 August 17, 2012) “While the incidence of new hepatitis C virus cases has decreased, the prevalence of infection will not peak until the year 2040. In addition, as the duration of infection increases, the proportion of new patients with cirrhosis will double by 2020 in
an untreated patient population.” (Rodriguez-Luna and Douglas 2004)
Screening
“Screening for HCV infection could identify persons at earlier stages of disease, before they develop serious or irreversible liver damage, and lead to treatments to improve clinical outcomes or reduce transmission risk. Up to three quarters of HCV-infected persons are unaware of their status.” (USPSTF Screening for HCV, Systematic Review 2012 http://www.uspreventiveservicestaskforce.org/uspstf12/hepc/hepcscrart.htm)
The CDC recommended HCV testing be initiated with an FDA – approved test for antibody HCV followed by an HCV nucleic acid test (NAT) for persons who test positive. (CDC MMWR Vol. 61/No. 4 August 2012) The USPSTF stated that anti-HCV antibody testing followed by polymerase chain reaction testing (a type of NAT) is accurate for identifying patients with chronic HCV infection. (USPSTF Screening for HCV, Systematic Review 2012 http://www.uspreventiveservicestaskforce.org/uspstf12/hepc/hepcscrart.htm)
Treatment
The goal of treatment of HCV in adults is to eradicate the virus and prevent long-term complications such as cirrhosis, liver failure and hepatocellular carcinoma (Chou, Hartung 2013). In the medical literature, 100% eradication of the virus from the blood is referred to as a sustained virologic response (SVR). Very recently, another type of genotype named IL-28B has been identified as a predictor of response to treatment. The presence of IL-28B is associated with a two-fold greater SVR in European, African American and Hispanic populations. Its presence or absence is used to determine whether to initiate treatment.
The standard treatment for many years consisted of a combination of pegylated interferon and ribavirin (a.k.a., dual therapy). However, this two-drug regimen leads to numerous side effects (Gravitz 2011), which can result in discontinuation of treatment prior to completing the full course. In addition, this regimen is less effective for genotype 1, which is the predominant genotype in the U.S. (about 75% of cases) and the more difficult genotype to treat. (Chou, Hartung 2013)
However, treatment of HCV for adults continues to evolve. In the past several years, FDA approved two protease inhibitors, boceprevir (Victrelis) and telaprevir (Incivek), for the treatment of genotype 1 infection. These antivirals are commonly referred to as direct acting antivirals (DAAs). Thus, the new standard of care for previously untreated (treatment naïve) patients with genotype 1 HCV infection is 12 – 32 weeks of boceprevir or telaprevir in combination with 24 – 48 weeks of pegylated interferon and ribavirin. (Lawitz 2013) The exact duration of treatment is dictated by the patient's response to therapy as well as the stage of hepatic fibrosis. (Lawitz 2013) The use of a DAA in combination with interferon and ribavirin is commonly referred to as triple therapy. On November 22, 2013 FDA approved a third antiviral called simeprevir (Olysio), which is a protease inhibitor indicated for HCV genotype 1 infection. Simeprevir is to be administered in combination with pegylated interferon and ribavirin.
(http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377234.htm)
On December 6, 2013 FDA approved sofosbuvir (Sovaldi), which is a nucleotide analog HCV NS5B polymerase inhibitor indicated for use for HCV infection due to genotypes 1, 2, 3 or 4. The FDA recommended regimens for patients with genotype 1 or 4 infection is sofosbuvir with pegylated interferon and ribavirin and for patients with genotype 2 or genotype 3 infection is sofosbuvir with ribavirin. (http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920.htm)
III. History of Medicare Coverage
Pursuant to §1861(ddd) of the Social Security Act, the Secretary may add coverage of "additional preventive services" if certain statutory requirements are met. Our regulations provide:
§410.64 Additional preventive services
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Services Task Force.
(3) Appropriate for individuals entitled to benefits under part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
Currently, screening for HCV is not covered by Medicare.
IV. Timeline of Recent Activities
| Date |
Action |
| September 5, 2013 |
CMS initiates opening a NCA for screening for HCV. Initial 30-day public comment period begins. |
| October 5, 2013 |
Initial public comment period closed.
|
| March 4, 2014 |
Posted PDM. Second 30-day public comment period begins. |
| April 3, 2014 |
Second public comment period closed.
|
V. Food and Drug Administration (FDA) Status
In general, diagnostic laboratory tests are regulated by the FDA. Numerous laboratory tests that can detect the presence of HCV antibody as well as HCV polymerase chain reaction tests are FDA approved/cleared and available. The FDA In Vitro Diagnostics database provides specific information on the approved or cleared tests.
VI. General Methodological Principles
When making national coverage determinations concerning additional preventive services, CMS applies the statutory criteria in §1861(ddd) of the Social Security Act and evaluates relevant clinical evidence to determine whether or not the service is reasonable and necessary for the prevention or early detection of illness or disability, is recommended with a grade of A or B by the USPSTF, and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B of the Medicare program.
Public comment sometimes cites the published clinical evidence and gives CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum.
VII. Evidence
A. Introduction
Consistent with §1861(ddd)(1)(A) and 42 CFR § 410.64(a)(1), additional preventive services must be reasonable and necessary for the prevention or early detection of illness or disability. With respect to evaluating whether screening tests conducted on asymptomatic individuals are reasonable and necessary for these purposes, the analytic framework involves consideration of different factors compared to either diagnostic tests or therapeutic interventions. Evaluation of screening tests has been largely standardized in the medical and scientific communities, and the "value of a screening test may be assessed according to the following criteria:
- Simplicity. In many screening programmes more than one test is used to detect one disease, and in a multiphasic programme the individual will be subjected to a number of tests within a short space of time. It is therefore essential that the tests used should be easy to administer and should be capable of use by para-medical and other personnel.
- Acceptability. As screening is in most instances voluntary and a high rate of co-operation is necessary in an efficient screening programme, it is important that tests should be acceptable to the subjects.
- Accuracy. The test should give a true measurement of the attribute under investigation.
- Cost. The expense of screening should be considered in relation to the benefits resulting from the early detection of disease, i.e., the severity of the disease, the advantages of treatment at an early stage and the probability of cure.
- Precision (sometimes called repeatability). The test should give consistent results in repeated trials.
- Sensitivity. This may be defined as the ability of the test to give a positive finding when the individual screened has the disease or abnormality under investigation.
- Specificity. This may be defined as the ability of the test to give a negative finding when the individual screened does not have the disease or abnormality under investigation." (Cochran and Holland 1971).”
As Cochrane and Holland (1971) noted, evidence on health outcomes, i.e., “evidence that screening can alter the natural history of disease in a significant proportion of those screened," is important in the consideration of screening tests since individuals are asymptomatic and "the practitioner initiates screening procedures.” (Cochran and Holland 1971)
Four of the seven criteria cited above (Cochrane and Holland 1971) as reasonable and necessary for screening tests (i.e., accuracy, precision, sensitivity and specificity) reflect a screening test's ability to minimize the harm of testing inaccuracy, especially from false positive or false negative results. Screening test compliance with these criteria is within the scope of FDA review of in-vitro diagnostic devices and the FDA has only reviewed evidence on the approved label indications for these tests.
Primary Care and USPSTF Recommended Preventive Services
The USPSTF functions as an independent panel of non-Federal experts in prevention and primary care. The primary audience for the USPSTF's work remains primary care clinicians. From the Preface to 2012 USPSTF Clinical Preventive Services Guide (available at http://www.ahrq.gov/clinic/pocketgd1011/pocketgd1011.pdf, pp. v-vi):
“Preface
Since being codified by Congress, the U.S. Preventive Services Task Force (USPSTF) has been fulfilling its charge to conduct rigorous reviews of scientific evidence to create evidence-based recommendations for preventive services that should be provided in the primary care setting.… Our Procedure Manual, which can be found at http://www.USPreventiveServicesTaskForce.org/uspstf08/methods/procmanual.htm, outlines our updated process for evaluating the quality and strength of the evidence for a service, determining the net health benefit (benefits minus harms) associated with the service, and judging the level of certainty that providing these services will be beneficial in primary care.”
The Institute of Medicine (IOM) has provided a definition of primary care based on the function which states: “Primary care is the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community (IOM. Primary Care: America's Health in a New Era 1996).” In discussing the value of primary care, one of the elements cited supporting this definition is that primary care “…opens opportunities for disease prevention and health promotion as well as early detection of disease….” (IOM Primary Care: America's Health in a New Era 1996) The Agency for Healthcare Research and Quality (AHRQ) has adopted the IOM definition of primary care in their primary care practice based research networks (PBRNs) (http://pbrn.ahrq.gov/portal/server.pt/community/practice_based_research_networks_%28pbrn%29__about/852).
Many preventive services are discussed within the context of the primary care setting and the USPSTF reviews preventive services that should be provided in the primary care setting. Primary care providers are thought of as the initial contact for patients within a complicated health system. Primary care providers are often identified as the conduit for identifying the need for preventive services by assessing the patient's individual risk factors and developing a comprehensive prevention plan that directs patients in a coordinated manner to appropriate services to address their individual health risks and provide the most efficient utilization of health care services.
B. USPSTF Grade Definitions
The U.S. Preventive Services Task Force (USPSTF) assigns one of five letter grades to each of its recommendations (A, B, C, D, I). In July of 2012, the grade definitions were updated to reflect the change in definition of and suggestions for practice for the grade C recommendation.
The following tables from the USPSTF website provide the current grade definitions and descriptions of levels of certainty. (http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm)
Grade Definitions After July 2012
| Grade |
Definition |
Suggestions for Practice |
| A |
The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
Offer or provide this service.
|
| B |
The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
Offer or provide this service. |
| C |
The USPSTF recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences. There is at least moderate certainty that the net
benefit is small. |
Offer or provide this service for selected patients depending on individual circumstances.
|
| D |
The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
Discourage the use of this service. |
| I Statement |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
Read the clinical considerations section of USPSTF Recommendation Statement. If the service is offered, patients should understand the uncertainty about the balance of benefits and harms. |
| Level of Certainty |
Description |
| High |
The available evidence usually includes consistent results from well-designed, well-conducted studies in representative primary care populations. These studies assess the effects of the preventive service on health outcomes. This conclusion is therefore unlikely to be strongly affected by the results of future studies. |
| Moderate |
The available evidence is sufficient to determine the effects of the preventive service on health outcomes, but confidence in the estimate is constrained by such factors as:
The number, size, or quality of individual studies.
Inconsistency of findings across individual studies.
Limited generalizability of findings to routine primary care practice.
Lack of coherence in the chain of evidence.
As more information becomes available, the magnitude or direction of the observed effect could change, and this change may be large enough to alter the conclusion.
|
| Low |
The available evidence is insufficient to assess effects on health outcomes. Evidence is insufficient because of:
The limited number or size of studies.
Important flaws in study design or methods.
Inconsistency of findings across individual studies.
Gaps in the chain of evidence.
Findings not generalizable to routine primary care practice.
Lack of information on important health outcomes.
More information may allow estimation of effects on health outcomes. |
* The USPSTF defines certainty as "likelihood that the USPSTF assessment of the net benefit of a preventive service is correct." The net benefit is defined as benefit minus harm of the preventive service as implemented in a general, primary care population. The USPSTF assigns a certainty level based on the nature of the overall evidence available to assess the net benefit of a preventive service.
C. U.S. Preventive Services Task Force Recommendations for HCV–(link provided http://www.uspreventiveservicestaskforce.org/uspstf12/hepc/hepcfinalrs.htm)
The USPSTF recommends screening for HCV infection in persons at high risk for infection. (Grade: B recommendation) The USPSTF also recommends offering 1-time screening for HCV infection to adults born between 1945 and 1965. (Grade: B recommendation).
“In 1998, the highest prevalence rates of the anti-HCV antibody occurred in persons with significant direct percutaneous exposures, such as injection drug users and persons with hemophilia (60% to 90%); persons with less significant percutaneous exposures involving smaller amounts of blood, such as patients receiving hemodialysis (10% to 30%), had more moderate prevalence rates. Persons engaging in high-risk sexual behaviors (1% to 10%); recipients of blood transfusions (6%); and persons with infrequent percutaneous exposures, such as health care workers (1% to 2%), had the lowest prevalence rates."
“In reviewing the prevalence data on high-risk groups and the potential for reduced transmission, the USPSTF concluded that screening in high-risk persons (prevalence ≥50%) and the birth cohort (prevalence of about 3% to 4%) would result in a moderate net benefit.”
The evidence supporting these recommendations is considered and summarized in section VII.E of this document.
D. Literature Search
In addition to the prerequisite USPSTF recommendations, CMS must consider not only whether an additional preventive service is reasonable and necessary for the prevention or early detection of illness or disability, but whether the service is appropriate for individuals entitled to benefits under part A or enrolled under part B of the Medicare program.
CMS performed its literature search using PubMed on December 16, 2013 with the search terms “mass screening,” “hepacivirus” and “human.” The following limitations were applied: English, Clinical Trial and a publication within the last ten years. Citations that presented the results of a clinical study were found and reviewed at the abstract level. Numerous studies in which HCV screening was an outcome, rather than an intervention, were judged not relevant to this topic. Four citations were included for further review in the evidence section of this PDM (Moyer 2013; Chou, Cottrell 2013; CDC MMWR Vol.61/No. 4 August 17, 2012; Coffin 2011). Moyer 2013 presents the most recent screening recommendation from the USPSTF. Chou, Cottrell 2013 presents a technology assessment funded by AHRQ. The CDC MMWR 2012 publication presents the recommendations of the CDC. Coffin 2011 presents the results of a cost-effectiveness analysis. An updated search was performed on April 11, 2014 and no new clinical evidence was identified.
In addition to the citations found through the literature search, two documents of interest were discovered upon examination of the bibliography of each citation mentioned above. (Liu 2013; Rein 2012) Finally, one practice guideline from the American Association for the Study for Liver Diseases was identified by a public commenter (Ghany 2009).
E. Discussion of Evidence
Our discussion focuses upon the adequacy of the evidence to draw conclusions about the risks and benefits of screening for HCV for adult Medicare patients, in other words, for those beneficiaries who are 65 years old and older and those who are on Medicare disability or in the End Stage Renal Disease (ESRD) program.
CMS searches for and considers literature articles, reports and guidelines that present evidence rather than present a review or a commentary. This evidence usually concerns clinical health outcomes associated with screening for HCV infection that typically are objective in nature, such as mortality and adverse event rates. Consequently, studies that evaluate screening test strategies are not as relevant and are less helpful to CMS. Lastly, when evaluating “additional preventive services,” CMS may conduct an assessment of the relation between predicted outcomes and the expenditures for the service. §1861(ddd)(2); 42 C.F.R. §410.64(b).
1 Questions:
The questions of interest for this national coverage analysis are:
1a. Is the evidence sufficient to determine that screening for hepatitis C virus infection in all persons at high risk for infection is recommended with a grade of A or B by the USPSTF?
1b. Is the evidence sufficient to determine that screening for hepatitis C virus infection in all persons at high risk for infection improves outcomes in the prevention or early detection of illness or disability?
1c. Is the evidence sufficient to determine that screening for hepatitis C virus infection in all persons at high risk for infection is appropriate for Medicare beneficiaries?
2a. Is the evidence sufficient to determine that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 is recommended with a grade of A or B by the USPSTF?
2b. Is the evidence sufficient to determine that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 improves outcomes in the prevention or early detection of illness or disability?
2c. Is the evidence sufficient to determine that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 is appropriate for Medicare beneficiaries?
2 External Technology Assessments (TA)
CMS did not commission an external TA on this topic.
3 Internal TA
Evidence Summary
Chou R, Cottrell EB, Wasson N, et al. Screening for hepatitis C virus infection in adults: A systematic review for the U.S. Preventative Services Task Force. Annuals of Internal Medicine 2013;158:101.
This article provides a synopsis of a systematic review prepared for the USPSTF by the Oregon Evidence Practice Center, which was under contract to AHRQ. The full report of this systematic review is found at http://www.effectivehealthcare.ahrq.gov/ehc/products/285/1283/CER69_HepatitisCScreening_FinalReport_20121015.pdf.
The authors performed a systematic review based on evidence obtained after a search of the medical literature dating from 1947 to May 2012, the Cochrane Library Database, clinical trial registries and reference lists. The review focused on HCV screening in asymptomatic pregnant or non-pregnant adults without known liver enzyme abnormalities and on "research gaps identified in the 2004 USPSTF review and new studies published since that review." Studies of post-transplant patients, HIV-infected patients, patients undergoing hemodialysis and/or people with occupational-related exposure to HCV were excluded. The goals of the review were:
- to determine if screening for HCV in the target population reduces mortality and morbidity due to HCV infection, affects quality of life or reduces incidence of HCV infection;
- to determine the effectiveness of different risk- or prevalence-based methods for screening on clinical outcomes;
- to determine the sensitivity and number needed to screen to identify one case of HCV infection of different risk- or prevalence-based methods for screening; and
- to assess the harms associated with screening.
Study selection criteria limited the review to evidence from randomized trials and cohort, case-control, and cross-sectional studies that assessed yield or clinical outcomes of HCV screening. The “analytic framework focuses on direct evidence that HCV screening improves important health outcomes compared with not screening, as well as the chain of indirect evidence (diagnostic accuracy of screening, clinical utility and harms of subsequent testing in HCV-infected persons, and benefits and harms of treatments) linking screening with improved health outcomes.” Health outcomes assessed were “mortality, end-stage liver disease, cirrhosis, hepatocellular carcinoma, need for transplantation, quality of life, HCV transmission, harms associated with screening (such as anxiety, labeling, and effects on quality of life), and harms associated with liver biopsy (including death, bleeding, and severe pain).” With studies that reported the diagnostic yield of different screening strategies, the authors "computed the number needed to screen to identify 1 case of HCV infection by dividing the number of screening tests performed by the number of HCV cases identified. The proportion screened was the number of patients screened upon application of a particular screening strategy, divided by the total number of patients assessed." The overall strength of the evidence was rated as “high,” “moderate,” “low,” or "insufficient” using AHRQ's methodology (AHRQ April 2012).
For goals one and two, the authors did not find any studies that "compared clinical outcomes between individuals screened and not screened for HCV infection or between individuals screened by using different risk- or prevalence-based strategies."
For goal three, data for determining the diagnostic accuracy and yield of risk-based screening methods were based on four cross-sectional studies that were rated "Fair" and one case-control study rated as "Poor." Two cross-sectional studies were conducted in sexually-transmitted disease clinics and the remaining cross-sectional studies were conducted in urban primary care clinics. A wide variety of screening strategies were examined within and across each study. The demographic profile of the study population for each study also varied widely. The authors did not perform a subset analysis based on age or report results based on age. However, the authors' evidence table for these five studies (as provided in the full report) indicated that for each study the majority of the patient population was less than 65 years of age.
The cross-sectional study with the lower-prevalence population "found that screening based on presence of 1 or more positive items on a 20-item questionnaire was associated with a sensitivity of 90% for identifying persons with HCV infection and a number needed to screen to identify 1 case of HCV infection of 2.4." The "Three cross-sectional studies in higher-prevalence populations found that screening strategies targeting multiple risk factors were associated with sensitivities of more than 90% and numbers needed to screen of 9.3 to 18." The authors noted that "More narrowly targeted screening strategies evaluated in these studies were associated with specificities of more than 95% and numbers needed to screen of less than 2, but missed up to two thirds of infected patients."
For goal four, the authors noted that "Data on direct harms of screening were sparse. A large study of percutaneous liver biopsies (n = 2740) in HCV-infected patients with compensated cirrhosis reported no deaths and a 1.1% rate of serious adverse events (primarily bleeding and severe pain)."
Limitations of the systematic review identified by the authors included a lack of modeling studies to be assessed and a lack of inclusion into calculations of screening yield of high or unreported proportions of potentially eligible patients in the observational studies due to unknown HCV status. In addition, the authors noted that "The CDC's birth cohort approach was not evaluated in the studies included in our review on the yield of alternative screening strategies. Clinical studies that prospectively evaluate the accuracy, yield, and outcomes of alternative HCV screening strategies, including the birth cohort approach, are needed."
The authors noted that similar to the 2004 USPSTF review, "we found no direct evidence on effects of HCV screening versus no screening on clinical outcomes, or on the comparison of clinical effects of alternative screening strategies." It was further noted that "In the absence of direct evidence on clinical outcomes associated with screening, an indirect chain of evidence showing the availability of accurate diagnostic tests and effective treatments could link screening with improvements in clinical outcomes. The 2004 USPSTF review found HCV antibody testing to be highly accurate. Much of the benefits from screening are likely to be based on the effectiveness of antiviral treatments, including newly approved direct-acting antiviral agents, which are addressed in a separate review. Therefore, screening recommendations should be based on the evidence for screening and treatment in totality. Studies showing that screening or subsequent interventions are associated with decreased transmission risk could also significantly affect estimates of potential benefits, but these are not yet available."
The authors concluded that “Although screening tests can accurately identify adults with chronic HCV infection, targeted screening strategies based on the presence of risk factors misses some patients with HCV infection. Well-designed prospective studies are needed to better understand the effects of different HCV screening strategies on diagnostic yield and clinical outcomes.”
Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for Hepatitis C Virus Infection in Adults: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine September 2013. Volume 159 • Number 5, Pages 349-358. (Summary of Evidence for USPSTF Recommendation for Screening)
The USPSTF presented its recommendation statement, which “applies to all asymptomatic adults without known liver disease or functional abnormalities,” in an article published in the medical literature. (Moyer 2013) The USPSTF stated in this article that:
"The USPSTF found adequate evidence that anti-HCV antibody testing followed by confirmatory polymerase chain reaction testing accurately detects chronic HCV infection."
"In screening strategies targeting persons with risk factors for HCV infection (such as past or present injection drug use, sex with an injection drug user, or blood transfusion before 1992), anti-HCV antibody testing is associated with high sensitivity (>90%) and small numbers needed to screen to identify 1 case of HCV infection (< 20 persons). Anti-HCV antibody testing remains highly accurate in low-prevalence populations, although the numbers needed to screen to detect 1 case of HCV infection are higher."
"The USPSTF also found adequate evidence that various noninvasive tests have good to very good diagnostic accuracy in diagnosing fibrosis or cirrhosis."
"The USPSTF found no direct evidence on the benefit of screening for HCV infection in asymptomatic adults in reducing morbidity and mortality. However, the USPSTF found adequate evidence that antiviral regimens result in sustained virologic response (SVR) and improved clinical outcomes."
"The USPSTF found inadequate evidence that counseling or immunization of patients with HCV infection against other infections improves health outcomes, reduces transmission of HCV, or changes high-risk behaviors. The USPSTF found inadequate evidence that knowledge of positive status for HCV infection reduces high-risk behaviors. The USPSTF also found inadequate evidence that labor management and breastfeeding strategies in HCV-positive women are effective at reducing risk for mother-to-child transmission."
“Given the accuracy of the screening test and the availability of effective interventions for HCV infection, the USPSTF concludes that screening is of moderate benefit for populations at high risk for infection. The USPSTF concludes that 1-time screening in all adults in the United States born between 1945 and 1965 is also of moderate benefit.”
“The USPSTF found limited evidence on the harms of screening for HCV. Potential harms of screening include anxiety, patient labeling, and feelings of stigmatization.”
“The USPSTF found adequate evidence on the harms associated with the diagnostic evaluation used to guide treatment decisions (liver biopsy). These harms include bleeding, infection, and severe pain in approximately 1% of persons who had a liver biopsy and death in less than 0.2%. However, the use of liver biopsy to guide treatment decisions is declining, and noninvasive tests have sufficient accuracy to diagnose fibrosis and cirrhosis. Thus, the absolute risk to persons who currently receive a diagnosis of HCV infection and subsequent treatment is probably declining.”
“The USPSTF found adequate evidence that antiviral therapy regimens are associated with a high rate of harms, such as fatigue, headache, flu-like symptoms, hematologic events, and rash. However, antiviral therapy is given for a defined duration, serious adverse events are uncommon, and adverse events are self-limited and typically resolve after treatment is discontinued. The USPSTF found adequate evidence that these harms of treatment are small.”
“The USPSTF concludes with moderate certainty that screening for HCV infection in adults at increased risk for infection and 1-time screening in adults in the 1945 – 1965 birth cohort has moderate net benefit.”
CDC MMWR - Smith BD, Morgan RL, Beckert GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945 – 1965. MMWR August 17, 2012. Vol. 61, No. 4, pages 1 - 32.
This report presents the CDC's recommendation to expand its 1998 HCV testing guidelines. The CDC formed a team of experts from within and outside the Federal Government called the HCV Birth Cohort Testing Work Group (Work Group). Using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework, the Work Group performed a comprehensive systematic review of the literature to determine the availability and quality of the evidence regarding HCV infection prevalence as well as the health benefits and harms associated with one-time HCV testing for persons unaware of their status. Outcomes of interest included all-cause mortality, HCC, SVR, serious adverse events (SAEs), quality of life (QoL) and HCV transmission. The CDC's Division of Viral Hepatitis developed the proposed and, after peer-review by external experts and a public comment period, the final recommendation.
The report noted that "CDC considered various birth cohorts with increased HCV prevalence. For each proposed birth cohort, CDC determined the weighted, unadjusted anti-HCV prevalence and the size of the population. On the basis of HCV prevalence and disease burden, the 1945 - 1965 birth cohort was selected as the target population." The prevalence of anti-HCV in this target population is 3.25%.
The authors noted that "evidence was found in the literature for all-cause mortality, HCC, SVR, SAEs, QoL" but not for HCV transmission. For the QoL outcome, the authors judged the quality of evidence to be "low" due to limited availability and study design.
For all-cause mortality, the authors identified 22 relevant articles however 21 of these did not meet the inclusion criteria due to "insufficient sample sizes, unrepresentative study population, and other sources of confounding." Only one (Backus 2011) was directly applicable to the targeted birth cohort. Backus 2011 reported "that treatment-related SVR was associated with a reduction in risk for mortality among persons who had HCV infection diagnosed (Relative risk [RR] = 0.45; 95% CI = 0.41 - 0.51)." However, the authors noted that "this study only compares persons who responded to therapy with those who did not respond and does not address a screened population or an untreated population. Differences in stage of liver disease between the groups had the potential to bias these finds, but those data were not available. Therefore, the confidence in the estimate of effect was deemed to be low, and no change in rating of the quality of evidence was performed despite a large estimated treatment effect."
The Working Group assessed 12 observational studies that investigated the incidence of HCC in individuals achieving an SVR compared to those who did not respond to treatment. The authors stated that "Treatment-related SVR was associated with a reduced risk for HCC (> 75%) among persons at all stages of fibrosis (RR = 0.24; 95% CI = 0.18 - 0.31)."
Regarding SVR, the authors noted that the combination of pegylated interferon plus ribavirin (PR) and first generation direct-acting antiviral agents (DAA) "increases the rate of SVR in treated persons with hepatitis C genotype 1 when compared with PR alone. Pooled estimates comparing boceprevir- and telaprevir-based regimens with PR suggest that these regimens are associated with 28% increases in SVR rates (RR = 0.28, 95% CI = 0.24 - 0.32). Although SVR was initially judged by the Work Group to be directly associated with patient-important outcomes (e.g., reduced viral transmission), further deliberation resulted in SVR being defined as an intermediary outcome that is predictive of a reduction in morbidity and mortality, particularly from HCC."
Regarding SAEs, the CDC report noted that "Treatment for HCV infection with PR can result in serious adverse events (SAEs). In May 2011, triple-drug therapy with PR and DAA became the standard of care for patients with HCV genotype 1, but limited data are available for systematic reviews on SAEs for regimens including these new agents." The authors added that "Although the addition of boceprevir and telaprevir to standard treatment with PR increases the rate of SVR in persons with HCV genotype 1, it also has been shown to result in an increased rate of adverse events that are severe enough to lead to treatment discontinuation (RR = 1.34; 95% CI = 0.95 - 1.87)."
The CDC concluded with a recommendation that “Adults born during 1945 – 1965 should receive one-time testing for HCV without prior ascertainment of HCV risk.” This recommendation was assessed as a "Strong Recommendation, Moderate Quality of Evidence."
Cost-effectiveness of HCV Screening
A number of authors have addressed the question of cost for HCV screening. Several of the most recent publications are presented below.
Liu SL, Cipriano LE, Holodniy M and Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United States. PLoS ONE 2013;8:e58975.
The authors performed decision analysis/Markov modeling to evaluate various screening strategies (including risk-based and birth cohort-based strategies) and various treatment strategies (including dual therapy, triple therapy and IL-28B guided therapy strategies) in asymptomatic 40 - 74 year old (base case age, 50 years) adults in the U.S. who were unaware of their HCV infection status. Cohorts were stratified by age, sex, race, risk history, HCV infection status, HCV genotype, treatment eligibility, IL-28B genotype and initial liver fibrosis stage. National Health and Nutrition Examination Survey (NHANES) data were used to provide prevalence of risk factors and mortality rates. Screening-related costs were estimated using the 2010 Medicare fee schedule. Costs were inflation-adjusted to 2010 U.S. dollars. The primary outcomes were lifetime costs and quality-adjusted life years (QALYs). A societal perspective was used.
The authors found that "In the base case, risk-factor guided and birth-cohort screening of individuals who are currently 50 years of age, respectively, averted 4 - 7 and 10 - 15 liver transplants, 13 - 27 and 35 - 56 liver cancers, and gained 181 - 450 and 483 - 950 QALYs per 100,000 people compared to no screening, depending on the HCV treatment strategy used and assuming 30 - 40% treatment uptake and 70% treatment adherence. Risk-factor guided and birth-cohort screening, respectively, increased costs by $17 - 30 million and $35 - 57 million per 100,000 people compared to no screening. Birth-cohort screening yielded greater health benefits per dollar spent than risk-factor guided screening in all cases largely because risk factors for HCV are too common and not sufficiently predictive. Compared to no screening, birth-cohort screening of individuals who are currently 50 years of age followed by IL-28B-guided triple-therapy costs $60,590 per QALY gained. Birth-cohort screening followed by universal triple therapy costs $65,749 per QALY gained." In addition, "Birth-cohort screening followed by universal triple therapy costs less than $100,000 per QALY for ages 40 - 64 years compared to the next best strategy." The results showed that "Lower levels of treatment uptake erode the cost-effectiveness of HCV screening. If only 10% of the screened and treatment-eligible population initiate treatment at each opportunity, birth-cohort screening with triple therapy costs $241,100 per QALY compared to no screening. Birth-cohort screening costs approximately $50,000 per QALY only when treatment uptake is greater than 50%." The authors did not report results specifically for patients 65 years of age or older.
The authors noted some limitations of their analysis including small sample sizes in the NHANES database, the exclusion of patients with hepatitis B or HIV infection and the impact of this exclusion on real-world situations, and the lack of "observational studies that provide long-term follow-up data on differential mortality rates among people in various risk groups and HCV infection statuses."
The authors concluded that the "cost-effectiveness of one-time birth-cohort hepatitis C screening for 40 - 64 year olds is comparable to other screening programs, provided that the healthcare system has sufficient capacity to deliver prompt treatment and appropriate follow-on care to many newly screen-detected individuals."
Coffin PO, Scott JD, Golden MR and Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clinical Infectious Diseases 2012;54:1259.
The authors performed decision analysis/Markov modeling to estimate the cost-effectiveness of one-time HCV screening compared to risk-factor based screening in individuals 20 - 69 years (base case age, 45 years) of age in the U.S. using NHANES data, 2010 practice guidelines, 2010 Medicare prices for screening costs and inflation adjustment to 2010 U.S. dollars for any other costs. A subanalysis was conducted to compare screening the 1945 - 1965 birth cohort (assuming 15% of this cohort was screened) to the general population. The assumption was made that all individuals were treatment naive, had genotype 1 HCV infection and received triple therapy. Liver fibrosis stage was taken into consideration but IL-28B genotype status was not. The primary outcomes were costs and lifetime QALYs. A societal perspective was used.
The following table presents the key results.
| Screening Modality |
% CHC initially detected |
% of all CHC cured |
Cost/CHC |
QALY/CHC |
Cost/QALY |
Incremental Cost/QALY |
| Risk-based screening |
50% |
10.9% |
$59,938 |
13.50 |
$4439 |
n/a |
| General population (15% screened) |
58% |
12.1% |
$60,269 |
13.54 |
$4450 |
$7900 over risk factor based screening |
| 1945 - 1965 birth cohort (15% screened) |
62% |
12.6% |
$60,180 |
13.56 |
$4438 |
$5400 over risk factor based screening
|
CHC = chronic hepatitis C
The authors did not report results specifically for patients 65 years of age or older.
The authors noted a number of limitations including the lack of incorporation into their modeling of the impact of newer diagnostic tests such as IL-28B genotype and liver imaging procedures. The preliminary examination of the newer antiviral treatments was also not incorporated into their modeling due to the limited clinical experience with these drugs and the rapidly changing treatment regimens.
The authors concluded that "the addition of one-time screening of the general adult US population for CHC would be cost-effective over the current practice of only screening high-risk individuals. Target age-based screening, equivalent to screening only high-risk birth cohorts in our model, may be more cost-effective than general population screening if implementation costs, pace of adoption by clinicians, and median age of diagnosis were similar. Because the cost of managing CHC increases as the disease progresses, from an economic perspective the optimal time to implement broadened screening is now. Similar to recent experience with human immunodeficiency virus, broadened screening is only the first step in a comprehensive public health effort: successfully limiting HCV-associated morbidity and mortality will require initiatives to identify infected persons and ensure their treatment."
Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Annals of Internal Medicine 2012;156:263.
The authors performed decision analysis/Markov modeling to estimate the cost-effectiveness of one-time screening for HCV in U.S. adults from the 1945 - 1965 birth cohort based on Meta-analysis of Histologic Data in Viral Hepatitis (METAVIR) scale units. Medical outcomes, costs and QALYs were estimated. Reimbursement costs were based on the Medicare fee schedule. A lifetime, societal perspective was used.
Results indicated "that compared with the current strategy of risk-based screening, birth-cohort screening followed by standard treatment reduced deaths by 82,300 at a cost of $15,700 per QALY gained (95% credible interval, $11,500 to $30,100). Incorporating new DAA treatments would prevent approximately 121,000 deaths compared with risk-based screening at a cost of $35,700 per QALY saved (95% credible interval, $28,200 to $47,000)." Moreover, "If fully implemented, birth-cohort screening in primary care would identify 808,580 new cases (85.9% of all undiagnosed cases in the birth cohort, compared with 21.0% under risk-based screening) at a screening cost of $2874 per new infection identified."
The authors noted a number of limitations with their analysis including the assumption that someone without insurance did not receive antiviral treatment as well as the need to use estimates (rather than definitive data) of DAA costs and effectiveness given the newness of these antiviral drugs in clinical practice.
The authors concluded that "Birth-cohort screening for HCV in primary care settings was cost-effective" and that it "seems to be a reasonable strategy to identify asymptomatic cases of HCV."
4. Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) Meeting.
CMS did not hold a MEDCAC meeting on this topic.
5. Evidence-based clinical guidelines
A number of evidence based guidelines were identified.
USPSTF
U.S. Preventive Services Task Force Recommendations for HCV (see Background Section and summary of Moyer publication above ).
CDC
In 2012 the CDC stated its current recommendations regarding the prevention and control of HCV infection and HCV-related chronic diseases (CDC MMWR/Vol. 61/No.4 August 17, 2012):
“Adults born during 1945 – 1965 should receive one-time testing for HCV without prior ascertainment of HCV risk.”
“HIV-infected patients should be tested routinely for evidence of chronic HCV infection. Initial testing for HCV should be performed using the most sensitive immunoassays licensed for detection of antibody to HCV (anti-HCV) in blood.”
“Routine HCV testing is recommended for
- Persons who ever injected illegal drugs, including those who injected once or a few times many years ago and do not consider themselves as drug users.
- Persons with selected medical conditions, including
- persons who received clotting factor concentrates produced before 1987;
- persons who were ever on chronic (long-term) hemodialysis; and
- persons with persistently abnormal alanine aminotransferase levels.
- Prior recipients of transfusions or organ transplants, including
- persons who were notified that they received blood from a donor who later tested positive for HCV infection;
- persons who received a transfusion of blood or blood components before July 1992; and
- persons who received an organ transplant before July 1992.
Routine HCV testing is recommended for persons with recognized exposures, including
- Health care, emergency medical, and public safety workers after needle sticks, sharps, or mucosal exposures to HCV-positive blood.”
American Academy of Family Physicians (AAFP)
In its clinical recommendation statement for HCV (accessed on November 12, 2013 at http://www.aafp.org/patient-care/clinical-recommendations/all/hepatitis.html), the AAFP stated:
“The AAFP recommends screening for hepatitis C virus (HCV) infection in adults at high risk, including those with any history of intravenous drug use or blood transfusions prior to 1992. (2013)”
American Association for the Study for Liver Diseases (AASLD)
In a practice guideline prepared in conjunction with the American College of Gastroenterology (ACG) and the Infectious Disease Society of America (IDSA) (Ghany 2009), the AASLD recommended that all persons “who are at risk should be tested for the presence of HCV infection.” AASLD noted the following adults are at risk and should be tested:
- “Persons who have injected illicit drugs in the recent and remote past, including those who injected only once and do not consider themselves to be drug users.
- Persons with conditions associated with a high prevalence of HCV infection including:
- Persons with HIV infection
- Persons with hemophilia who received clotting factor concentrates prior to 1987
- Persons who have ever been on hemodialysis
- Persons with unexplained abnormal aminotransferase levels
- Prior recipients of transfusions or organ transplants prior to July 1992 including:
- Persons who were notified that they had received blood from a donor who later tested positive for HCV infection
- Persons who received a transfusion of blood or blood products
- Persons who received an organ transplant
- Health care, emergency medical and public safety workers after a needle stick injury or mucosal exposure to HCV-positive blood
- Current sexual partners of HCV-infected persons”
AASLD assigned a grade of “IB” to this recommendation, where a “I” stands for “Conditions for which there is evidence and/or general agreement that a given diagnostic evaluation procedure or treatment is beneficial, useful, and effective” and Level B stands for “Data derived from a single randomized trial, or nonrandomized studies.” A search of the AASLD website on November 12, 2013 did not find a more recent practice guideline.
6. Public Comments
Public comments sometimes cite the published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum.
Initial Public Comments
CMS received sixty-five comments. Sixty-three commenters advocated coverage for screening for HCV. A summary of these comments can be found in the proposed decision memorandum and the complete text of these comments is available on the CMS website at http://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=272.
Public Comments on Proposed Decision Memorandum
CMS received 23 comments on the PDM for screening for HCV in adults. All the commenters supported expanding coverage for screening for HCV. Of the 23 comments, one was from the manufacturer of a point of service test, three were from pharmaceutical companies and nine were from national organizations. While all of the commenters supported the coverage of screening for HCV, many of them expressed concern regarding some of the conditions for coverage. At least one commenter noted that the proposed decision was very thorough and comprehensive. The issues of concern expressed by the commenters are summarized below.
Extend Coverage Beyond Primary Care
Comments: A number of commenters requested that screening for HCV be extended to, among others, emergency departments, convenient care units, inpatient hospitals, ambulatory surgical centers, public entities (i.e. District Health Departments), infectious diseases specialists, GI specialists, or any appropriate Medicare-eligible clinician in any appropriate setting. One of the commenters referenced data from NHANES suggesting that > 50% of persons born between 1945 and 1965 are unaware of their HCV status and that those unaware are more likely to lack a “usual source of medical care.” Some commenters expressed the opinion that coverage should be extended beyond primary care because many individuals do not access the health care system through a primary care setting. One commenter stated that infectious diseases specialists were well-suited to initiate an evidence-based treatment approach as, necessary following screening. One commenter expressed concern about narrowing coverage decisions to tests ordered by primary care physicians or practitioners in primary care settings. The commenter stated there was no meaningful rational in the current PDM to support this requirement.
One commenter agreed with CMS regarding the important role of primary care practitioners in screening individuals for HCV and coordinating appropriate follow-up care and treatment but then went on to urge CMS to expand coverage to screening performed by practitioners in settings outside of primary care. Another commenter supported CMS' decision to cover HCV screening when ordered by a primary care provider or practitioner within the context of a primary care setting.
Response: CMS appreciates the concerns expressed by these commenters. While CMS is providing coverage for additional preventive services, we believe it is important that these preventive services should be provided in a coordinated approach as part of a comprehensive prevention plan within the context of the patient's total health care. Primary care practitioners are characterized by their coordination of a patient's comprehensive healthcare needs. Primary care practitioners are generalists who are specifically trained to provide primary care services. Other provider specialties may provide patient care in other settings but do not offer care in the context of being the coordinator of the patient's healthcare needs, not limited by problem origin or diagnosis. Coordination of health services is especially important in the presence of the coexisting health issues of our Medicare beneficiaries. Given the number and availability of primary care practitioners and settings, we believe that Medicare beneficiaries will have ready access to the additional preventive service established under this NCD.
Screening for HCV is recommended for persons who are asymptomatic and fall within the birth cohort or are determined to be at high risk. Everyone being screened is unaware of their status until the screening is completed.
As we state in section VII of this decision memorandum, the USPSTF conducts rigorous reviews of the evidence to create evidence-based recommendations for preventive services in the primary care setting. The primary audience for the USPSTF's work are primary care clinicians. Conceivably, state and local health clinics, family planning clinics, or other specialists, if they are functioning as the primary care provider/practitioner for a Medicare beneficiary, could be eligible to order screening for HCV if they meet the definition of primary care setting provided in this NCD and are eligible Medicare providers. This NCD requires that the HCV screening test be ordered by the primary care provider/practitioner. This NCD does not require the testing be done by the primary care provider/practitioner.
CMS believes that primary care practitioners are on the front lines of health care in providing prevention services. As preventive services gain recognition of their potential to improve the health status of the individual, we believe the primary care provider is in the unique position to provide a comprehensive and coordinated approach to Medicare beneficiaries' health care and this coordinated approach will help ensure the best outcomes for these services.
In addition, this coverage decision for screening for HCV in adults is for Medicare beneficiaries, of which many have comorbid conditions that require multiple interactions with the healthcare system. Thus, primary care practitioners may need to carefully evaluate the results of the screening test in determining the patient's overall treatment plan. In addition, since this is a one-time screening benefit for most patients, it becomes more important to coordinate care to and avoid the unnecessary duplication and non-coverage of excessive screening tests.
Comment: One commenter expressed that the limitation of coverage to screenings recommended by primary care practitioners in a primary care setting was inconsistent with Medicare regulations permitting the provision of preventive health assessments and care plan development in a broader array of settings. They stated that the initial preventive physical exam (IPPE) or subsequent annual wellness visits (AWV) are not limited to primary care providers or in primary care settings. They further stated that the Secretary's “authority to authorize NCDs to designate “additional preventive services” is not limited to authorization of services performed only in a primary care setting.”
Response: We do not agree that our requirement that the screening test must be ordered by primary care practitioners in a primary care setting is inconsistent with the Medicare regulations for additional preventive services codified at 42 C.F.R. § 410.64. It is true that the IPPE and AWV are established by separate statutes and regulations that have different requirements with respect to the suppliers that can furnish those services. See 42 C.F.R. §§ 410.16, 410.15 respectively. While some preventive services, such as the IPPE and AWV are specifically provided for in statute, screening for HCV in adults is established through the NCD process pursuant to §1861(ddd) of the Social Security Act. As we have explained previously, CMS may add coverage of "additional preventive services" if certain statutory requirements are met. We have further explained our reasoning for the requirement that the screening for HCV be ordered by a primary care practitioner within the context of a primary care setting in our responses to comments and in the analysis section of the decision memorandum.
Comment: One commenter expressed concern about the primary care requirement used in earlier national coverage determinations that are not currently being reconsidered and do not relate to hepatitis C screening in adults. Some other commenters identified other coverage for screening (e.g., screening for prostate cancer) that did not include the primary care requirement.
Response: Public comments about previous “additional preventive services” NCDs that are not currently being reconsidered are not within the scope of the current decision. Prostate cancer screening tests were established by statute under part B (§ 1861(oo)) and are not “additional preventive services.” Our regulations do establish qualifications for physicians and other practitioners for prostate cancer screening services that require that the physician or specified practitioner be “fully knowledgeable about the beneficiary” and be responsible for explaining the results of the screening examination or test. 42 C.F.R. § 410.39(a)(3). Although different language is used in the regulation, the requirement of a primary care physician or practitioner in a primary care setting in this NCD serves a similar function.
Other General Issues
Comments: The manufacturer of a rapid HCV test expressed concern about the terminology used in the decision memorandum. The manufacturer suggested the language might confuse providers and third-party payers and could limit access to screening for vulnerable populations. Specifically, the manufacturer requested that “point-of-care, or other” be added to the statement of coverage for screening tests in the decision memorandum. This commenter referenced the FDA approved point-of care tests. Another commenter also referenced the recently approved FDA point-of-care tests for HCV and urged CMS to finalize a NCD that ensures all forms of HCV blood test screening in adults are covered by Medicare. A second commenter also asked that CMS clarify that the HCV rapid tests are covered under the NCD.
Response: Given that the number of FDA approved tests could change over time, we are not adopting the commenter's suggestion that specific tests be identified in the NCD. Thus, the NCD does not address specific tests used to screen for HCV. We do not believe the word “laboratory” creates confusion, so we are not adopting the commenter's suggestion. It is consistent with the terminology used in a previous NCD for screening for sexually transmitted infections when the test used for screening was a blood test. The word is used to more clearly describe the screening test not where it may be performed.
Comments: Two commenters requested that CMS include information about this HCV screening benefit in the “Welcome to Medicare” packet received by new enrollees.
Response: We appreciate the suggestions. CMS routinely updates the informational and educational materials provided to our beneficiaries and providers to reflect the benefits that are available under the Medicare program.
Comments: One commenter recommended the use of one CPT code to reflect the provision of the HCV screening. The commenter expressed that this code would ensure accurate and appropriate billing for health care providers. Another commenter suggested that a G code be created.
Response: We appreciate the comment. This NCD does not address specific coding and billing instructions for this service. The NCD establishes conditions for coverage.
Comments: One commenter was concerned about the definition of “high risk” as it refers to “illicit” injection drug use. The commenter expressed that the use of the term “illicit” had a negative connotation and stigma attached to it and suggested the language be changed to remove the word “illicit”. Other commenters requested that CMS align our definition of “high risk” with the USPSTF or CDC recommendations.
Response: We are not adopting the commenters' suggestions. CMS believes the definition of “high risk” in the NCD is aligned with the USPSTF recommendation. While the USPSTF review identifies a number of risk factors, they identify high-risk as those with a HCV antibody prevalence of >50 percent. The CMS definition of high-risk in this NCD is consistent with USPSTF.
We are continuing to include the term “illicit” in our NCD in the phrase “current or past history of illicit injection drug use.” We believe that this limitation is necessary in defining the population at high risk. While the USPSTF recommendation does not specifically state “illicit” drug users, a number of the evidentiary references they cite in their bibliography do use the term “illicit” (Hagan 2008; Garland 1998). In addition, the CDC guidelines uses the term “illegal” as it pertains to drug use and the AASLD guidelines uses the term “illicit drugs”. There are a number of situations when beneficiaries use prescribed injectable drugs (e.g., insulin) and these individuals would not be considered at high-risk for HCV infection. We believe the term “illicit” clearly describes the individuals who are truly at high risk.
Comment: One commenter requested that CMS clarify that the FDA in Vitro Diagnostics database includes all FDA cleared and approved tests.
Response: CMS provides the link to the FDA in Vitro Diagnostics database as a convenience for the reader.
Comment: One commenter requested confirmation that coverage is for screening specifically and that follow-up confirmation testing (i.e., nucleic acid tests) is addressed under a separate coverage decision.
Response: The commenter is correct that our current NCD addresses only screening. When testing is needed to confirm the positive result of one test, the confirmatory test would be considered a diagnostic test and not screening. Coverage decisions concerning diagnostic testing will be made separately and are usually made by Medicare contractors.
There were numerous references provided by the commenters which are listed in Appendix A. The submitted references were reviewed and, if determined to be relevant to this NCA, are listed in the bibliography.
VIII. Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act. §1869(f)(1)(B). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Since January 1, 2009, CMS is authorized to cover "additional preventive services" (see Section III above) if certain statutory requirements are met as provided under §1861(ddd) of the Social Security Act. Our regulations at 42 CFR 410.64 provide:
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Service Task Force.
(3) Appropriate for individuals entitled to benefits under part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
CMS notes that any effect of the use of these screening tests is their coordination with treatment. CMS concludes that FDA approval or clearance of screening tests used consistent with FDA approved labeling provides a greater likelihood that a potential harm of screening testing, that is, taking action based on inaccurate screening test results, can be avoided. We further conclude that compliance by testing laboratories with CLIA regulatory requirements provides an additional, on-going safeguard for screening test quality. CMS considers these conditions essential to maximize patient safety.
In addition, CMS acknowledges that the USPSTF is charged with conducting rigorous reviews of scientific evidence to create evidence-based recommendations for preventive services that should be provided by primary care physicians and practitioners in primary care settings. In addition, the USPSTF Procedure Manual outlines the process for evaluating the quality and strength of the evidence for a service, determining the net health benefit (benefits minus harms) associated with the service, and judging the level of certainty that providing these services will be beneficial in primary care.
Evidence for Screening for HCV
Over time, HCV infection may progress to serious and potentially life-threatening complications including cirrhosis, liver failure, HCC and death. The USPSTF noted that HCV infection “is the leading cause of complications from chronic liver disease, and HCV-related end-stage liver disease is the most common indication for liver transplants among U.S. adults. It is estimated that the total number of patients with cirrhosis will peak at 1 million in 2020; however, rates of hepatic decompensation and liver cancer are expected to increase for another 10 to 13 years because of the lengthy lag time between infection and development of cirrhosis and other complications.” (Moyer 2013)
CMS notes that a recent systematic review of the benefits and harms of HCV screening in U.S. adults who are asymptomatic indicated limited evidence to directly link HCV screening with any health outcomes of importance to CMS. (Chou, Cottrell 2012) However, both the authors of this systematic review as well as the USPSTF (Moyer 2013) determined that there is adequate evidence of an indirect link between health outcomes and the sustained viral response seen during post-screening antiviral treatment of persons who are HCV-positive. Sustained viral response, which is defined as the complete eradication of the virus from the blood, is the goal of treatment. (Chou, Hartung 2013) The subsequent conclusions and recommendations of the USPSTF are based on the presence of this indirect link (Moyer 2013) and are supported by the CDC (CDC MMWR Vol.61/No. 4 August 17, 2012) as well as a number of evidence-based guidelines from professional societies. (Ghany 2009; AAFP 2013) Given that rates of hepatic decompensation and liver cancer are expected to continue increasing even once a peak incidence in cirrhosis is expected to be reached in 2020 (Moyer 2013), the implementation of screening to identify HCV-positive persons followed by the prompt administration of antiviral treatment stands to significantly impact health outcomes.
CMS acknowledges that there are real and potential harms associated with screening for HCV. These harms include anxiety and a decreased quality of life due to the knowledge of disease with the possibility of disease progression to liver failure, cancer and even death. (Moyer 2013) Additional harms are known to be associated with subsequent procedures performed to assess the clinical status of infection, although these harms are most often associated with invasive procedures that are less commonly in use. Side effects during antiviral treatment are also well documented (Chou, Cottrell 2012) and can lead to early discontinuation of treatment. The long-term potential harms are still to-be-determined for the newest antiviral drugs. (Chou, Cottrell 2012).
In general, tests do not directly exert an inherent therapeutic effect. In this case, the improved outcomes for beneficiaries infected with Hepatitis C accrue from the use of the test result to inform the subsequent use of therapies and not as a direct result of the act of testing. Thus we look for evidence establishing that the beneficiary's treating physician/practitioner uses a test result to recommend treatments that improve clinically meaningful health outcomes. We recognize that the avoidance of futile and harmful treatments may contribute to improved health outcomes.
Screening for High Risk Population
1a. Is the evidence sufficient to determine that screening for hepatitis C virus infection in all persons at high risk for infection is recommended with a grade of A or B by the USPSTF?
USPSTF Summary of Recommendation (2013)
“The USPSTF recommends screening for hepatitis C virus (HCV) infection in persons at high risk for infection.” (Grade: B recommendation)
CMS concludes that screening for hepatitis C virus infection in all persons at high risk for infection is recommended with a grade of A or B by the USPSTF.
1b. Is the evidence sufficient to determine that screening for hepatitis C virus infection in all persons at high risk for infection improves outcomes in the prevention or early detection of illness or disability?
CMS recognizes that the hepatitis C virus is the “most common chronic bloodborne pathogen in the U.S.” (Moyer 2013) According to the USPSTF, the “most important risk factor for HCV infection is past or current injection drug use, with most studies reporting a prevalence of 50% or more.” (Moyer 2013) In addition, “60% of new HCV infections occur in persons who report injection drug use within the past 6 months.” (Moyer 20132) Some of the evidence based guidelines specifically identify illicit or illegal injected drug use as the behavior that is considered high risk for infection. (CDC guidelines and AASLD guidelines) Additional risk factors include long-term hemodialysis, history of hemophilia, history of blood transfusion before 1992, getting an unregulated tattoo, and other percutaneous exposures (such as in health care workers). (Moyer 2013)
In the USPSTF recommendation statement, in updating the previous recommendation, the USPSTF described the high-risk group as a HCV antibody prevalence of > 50%. The USPSTF identified the prevalence of the different risk groups as: injection drug users and persons with hemophilia (60% to 90%); patients receiving hemodialysis (10% to 30%); persons engaging in high-risk sexual behavior (1% to 10%); recipients of blood transfusions (6%); and persons with infrequent exposures, such as health care workers (1% to 2%). (USPSTF Screening for Hepatitis C Virus Infection in Adults, Recommendation Statement 2013) Thus, CMS concludes the high-risk population is best described as the injection drug users (current or past) and persons who had a history of blood transfusions or blood product transfusions prior to 1992.
CMS notes that the USPSTF concluded “with moderate certainty” that HCV screening in adults at increased risk for infection has “moderate net benefit.” (Moyer 2013) However, as Moyer 2013 noted, there is “No evidence about how often screening should occur for persons who continue to be at risk for new HCV infection.” Neither the CDC (CDC MMWR Vol.61/No. 4 August 17, 2012; CDC 1998) nor the clinically-based guidelines from the professional societies addressed the issue of screening frequency in this particular population except to recommend “routine” screening; however, “routine” is not defined. In general, screening is only recommended if treatment of the disease at an earlier stage is more beneficial, in terms of health outcomes, than at a later stage (Wilson and Jungner 1968). Since the USPSTF recommendation and CDC evidence-based guidelines recommend screening for hepatitis C for individuals at high risk of infection, it is implicit that an appropriate treatment is available for these individuals. Since there is no guidance on the frequency of screening for the high risk population, CMS has determined that repeated screening would be appropriate annually for beneficiaries with continued high risk behavior, illicit injection drug use, since the previous negative screening test.
Results from cost-effectiveness studies generally support the USPSTF conclusion. (Liu 2013; Coffin 2012; Rein 2012) Of note, both Liu 2013 and Coffin 2012 noted that cost-effectiveness is dependent on the capacity of the healthcare system, particularly with regards to the pace of adoption of the HCV recommendations by healthcare professionals, the delivery of prompt and effective antiviral treatment and access to appropriate post-diagnostic, non-antiviral interventions.
After careful review of the available body of evidence, we conclude that screening for hepatitis C virus infection in all persons at high risk for infection provides improved outcomes in the prevention or early detection of illness or disability.
1c. Is the evidence sufficient to determine that screening for hepatitis C virus infection in all persons at high risk for infection is appropriate for Medicare beneficiaries?
The majority of the Part B Medicare beneficiary population is comprised of persons aged 65 years and older while a smaller percentage of the beneficiary population is comprised of the disabled, and those persons in the ESRD program. We note that the non-birth cohort-related USPSTF recommendation for this screening preventive service is based on risk factors and therefore would be applicable to any Medicare beneficiary, regardless of age. CMS notes that the USPSTF, CDC and professional societies do not specifically address the various aspects of screening for the 65 year old and older population.
CMS believes that the screening for HCV infection provides an opportunity for appropriate interventions to benefit the infected person by permitting for the early detection of, and potentially the prevention of, HCV-related liver disease. While the number of beneficiaries 65 years and older enrolled in the published studies is relatively small, CMS believes that the results are generalizable to the 65 years and older population.
After careful review of the available body of evidence, we conclude that screening for hepatitis C virus infection in all persons at high risk for infection is appropriate for Medicare beneficiaries.
Screening for Birth Cohort
2a. Is the evidence sufficient to determine that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 is recommended with a grade of A or B by the USPSTF?
USPSTF Summary of Recommendation (2013)
The USPSTF “recommends offering 1-time screening for HCV infection to adults born between 1945 and 1965.” (Grade: B recommendation)
CMS concludes that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 is recommended with a grade of A or B by the USPSTF.
2b. Is the evidence sufficient to determine that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 provides improved outcomes in the prevention or early detection of illness
or disability?
CMS recognizes that persons in the 1945 - 1965 birth cohort “are more likely to be diagnosed with HCV infection, possibly because they received blood transfusions before 1992 or have a history of other risk factors for exposure decades earlier.” (Moyer 2013) CMS also acknowledges that many of these persons may be asymptomatic and thus unaware that they are infected with the virus. As noted by the USPSTF, “A risk-based approach may miss detection of a substantial proportion of HCV-infected persons in the birth cohort because of a lack of patient disclosure or knowledge about prior risk status.” (Moyer 2013) About 75% of persons in the U.S. living with HCV infection are in the 1945 – 1965 birth cohort; the peak prevalence is 4.3% in persons who were 40 to 49 years old during 1999 – 2002. (Moyer 2013)
CMS notes that the USPSTF concluded “with moderate certainty” that one-time screening for HCV in adults in the 1945 – 1965 birth cohort has “moderate net benefit.” (Moyer 2013) Since the USPSTF recommendation and CDC evidence-based guidelines recommend screening for hepatitis C for individuals born from 1945 - 1965, it is implicit that an appropriate treatment is available for these individuals. Results from cost-effectiveness studies generally support the USPSTF conclusion. (Liu 2013; Coffin 2012; Rein 2012) Of note, Rein et al. found that “If fully implemented, birth-cohort screening in primary care would identify 808,580 new cases (85.9% of all undiagnosed cases in the birth cohort, compared with 21.0% under risk-based screening).”
After careful review of the available body of evidence, we conclude that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 provides improved outcomes in the prevention or early detection of illness or disability.
2c. Is the evidence sufficient to determine that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 is appropriate for Medicare beneficiaries?
The majority of the Part B Medicare beneficiary population is comprised of persons aged 65 years and older while a smaller percentage of the beneficiary population is comprised of the disabled, and those persons in the end-stage renal disease (ESRD) program. We note that as of the date of this final decision memorandum the majority of persons in the 1945 - 1965 birth cohort have yet to reach the age of 65 years. However, about 75% of persons in the U.S. living with HCV infection are in this birth cohort with a peak prevalence of 4.3% in persons who were 40 to 49 years old during 1999 – 2002. (Moyer 2013) Thus, a significant number of currently infected but asymptomatic future Medicare beneficiaries over time may have a progression of their HCV infection to serious and potentially life-threatening complications including cirrhosis, liver failure, HCC and death. The USPSTF noted that HCV infection “is the leading cause of complications from chronic liver disease, and HCV-related end-stage liver disease is the most common indication for liver transplants among U.S. adults. It is estimated that the total number of patients with cirrhosis will peak at 1 million in 2020; however, rates of hepatic decompensation and liver cancer are expected to increase for another 10 to 13 years because of the lengthy lag time between infection and development of cirrhosis and other complications.” (Moyer 2013) CMS notes that the USPSTF, CDC and professional societies do not specifically address the various aspects of screening for the 65 year old and older population.
We acknowledge the limited evidence concerning health outcomes of HCV screening. However, CMS believes that screening for HCV infection provides an opportunity for appropriate interventions to benefit the infected person by permitting for the early detection of, and potentially the prevention of, HCV-related liver disease. While the number of beneficiaries 65 years and older enrolled in the published studies is relatively small, CMS believes that the results are generalizable to the 65 years and older population. In general, screening is only recommended if treatment of the disease at an earlier stage is more beneficial, in terms of health outcomes, than at a later stage (Wilson and Jungner 1968).
After careful review of the available body of evidence, we conclude that 1-time screening for hepatitis C virus infection in all adults born between 1945 and 1965 is appropriate for Medicare beneficiaries.
Primary Care and USPSTF Recommended Preventive Services
CMS believes the primary care setting and the primary care provider are integral in the coordination of preventive services. The USPSTF creates evidence-based recommendations for preventive services that should be provided in the primary care setting by evaluating the quality and strength of the evidence for a service, determining the net health benefit (benefits minus harms) associated with the service, and judging the level of certainty that providing these services will be beneficial in primary care. (2012 USPSTF Clinical Preventive Services Guide)
CMS believes that preventive services should be provided within the context of a coordinated prevention plan based on the individual patient's needs assessed over time through the ongoing relationship established with the primary care provider. The IOM provides a definition of primary care (IOM. Primary Care: America's Health in a New Era 1996) and existing sections of the Social Security Act (§1833(u)(6), §1833(x)(2)(A)(i)(I) and §1833(x)(2)(A)(i)(II)) further defines primary care practitioners. The IOM further identifies one of the values of primary care as the opportunity for disease prevention and health promotion.
Based on the charge of the USPSTF in evaluating services provided in the primary care setting, CMS concludes referrals for the USPSTF recommended screenings for HCV for the specific populations should be ordered by the beneficiary's primary care provider in the primary care setting. CMS also believes that the IOM definition of primary care and the role of primary care in disease prevention and health promotion certainly supports that the risk assessment and referral for these screening services is best coordinated by the primary care provider in the primary care setting.
CMS concludes that the integrated and efficient utilization of these screening tests are best coordinated by the beneficiary's primary care provider and based on an evaluation of the patient risk factors and the appropriate referral and/or initiation of treatment. We are not indicating that the test itself must be performed by a primary care practitioner.
Disparities in HCV
“Improving health care disparities is an integral part of improving health care quality.” (AHRQ Healthcare Disparities Report, 2008)
Despite the overall success in the public health campaign against viral hepatitis, “there remain racial, ethnic, and socioeconomic disparities in the incidence and prevalence of acute and chronic viral hepatitis, the outcomes of chronic viral hepatitis, and health care access and quality.” (El-Serag 2010) As noted by USPSTF, “In 2010, the overall incidence rate of acute HCV infection was 0.3 cases per 100,000 persons and varied by race or ethnicity. The incidence rate for acute hepatitis C was lowest among persons of Asian or Pacific Islander descent and highest among American Indians and Alaskan natives. Blacks had the highest mortality rates from HCV, at 6.5 to 7.8 deaths per 100,000 persons, according to data from 2004 to 2008.” (Moyer 2013)
As stated in the National Plan for Action, from the National Partnership for Action, “ Beyond the heavy burden that the health and healthcare disparities represent for the individuals affected, there are additional social and financial burdens borne by the country as a whole. These burdens constitute both ethical and practical mandates to reduce health disparities and achieve health equity.” (The National Plan for Action Draft as of February 17, 2010)
Summary
HCV infection continues to be problematic in the U.S. with both health and societal consequences. The USPSTF reviewed the available evidence and provided a B recommendation for screening for HCV infection in adults at increased risk for infection and 1-time screening in adults in the 1945 – 1965 birth cohort. CMS reviewed the USPSTF recommendations and performed its own review of the evidence. The evidence supported that screening HCV infection in the USPSTF-indicated populations provided improved health outcomes with the assumption that the person receives appropriate post-screening treatment and/or non-pharmaceutical interventions.
Screening for HCV infection provides direct benefit to the Medicare beneficiary. Test results inform the treatment of an existing infection and such treatment can also prevent future health consequences.
CMS concludes the high risk population is best described as illicit injection drug users (current or past) and persons who have a history of receiving a blood transfusion prior to 1992. In addition, CMS concludes that repeated screening would be appropriate annually for beneficiaries with continued high risk behavior, e.g. needle sharing and/or syringe sharing, since the previous negative screening test. CMS also concludes that referral for screening for HCV infection is best ordered by the beneficiary's primary care provider within the context of a primary care setting.
CMS concludes that a FDA approved/cleared test, when used consistent with the FDA approved label, provides a greater likelihood that a potential harm of screening testing, i.e., taking action based on inaccurate screening test results, can be avoided. We further conclude that compliance by testing laboratories with CLIA regulatory requirements provides an additional, on-going safeguard for screening test quality. CMS considers these conditions essential to maximize patient safety.
IX. Conclusion
The CMS has determined the following:
The evidence is adequate to conclude that screening for HCV, consistent with the grade B recommendations by the USPSTF, is reasonable and necessary for the prevention or early detection of an illness or disability and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B, as described below.
Therefore, CMS will cover screening for HCV with the appropriate FDA approved/cleared laboratory tests, used consistent with FDA approved labeling and in compliance with the CLIA regulations, when ordered by the beneficiary's primary care physician or practitioner within the context of a primary care setting, and performed by an eligible Medicare provider for these services, for beneficiaries who meet either of the following conditions.
- A screening test is covered for adults at high risk for Hepatitis C Virus infection. “High risk” is defined as persons with a current or past history of illicit injection drug use; and persons who have a history of receiving a blood transfusion prior to 1992. Repeat screening for high risk persons is covered annually only for persons who have had continued illicit injection drug use since the prior negative screening test.
- A single screening test is covered for adults who do not meet the high risk as defined above, but who were born from 1945 through 1965.
The determination of “high risk for HCV” is identified by the primary care physician or practitioner who assesses the patient's history, which is part of any complete medical history, typically part of an annual wellness visit and considered in the development of a comprehensive prevention plan. The medical record should be a reflection of the service provided.
For the purposes of this decision memorandum, a primary care setting is defined by the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community. Emergency departments, inpatient hospital settings, ambulatory surgical centers, independent diagnostic testing facilities, skilled nursing facilities, inpatient rehabilitation facilities, clinics providing a limited focus of health care services, and hospice are examples of settings not considered primary care settings under this definition.
For the purposes of this decision memorandum, a “primary care physician” and “primary care practitioner” will be defined consistent with existing sections of the Social Security Act (§1833(u)(6), §1833(x)(2)(A)(i)(I) and §1833(x)(2)(A)(i)(II)).
§1833(u)
(6) Physician Defined.—For purposes of this paragraph, the term “physician” means a physician described in section 1861(r)(1) and the term “primary care physician” means a physician who is identified in the available data as a general practitioner, family practice practitioner, general internist, or obstetrician or gynecologist.
§1833(x)(2)(A)(i)
(I) is a physician (as described in section 1861(r)(1)) who has a primary specialty designation of family medicine, internal medicine, geriatric medicine, or pediatric medicine; or
(II) is a nurse practitioner, clinical nurse specialist, or physician assistant (as those terms are defined in section 1861(aa)(5));
Appendix A
The following are references submitted in comments as submitted by the commenter.
1 See e.g., The American Association for the Study of Liver Disease and Infectious Disease Society of America, “HCV Testing and Linkage to Care,” available at: http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care (last visited Mar. 31, 2014); and Centers for Disease Control, “Who Is At Risk for Hepatitis C?,” available at: http://www.cdc.gov/hepatitis/c/cfaq.htm (last visited Mar. 31, 2014).
1 Arora S, Thornton K, Murata, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011; 364:2199-2207.
2 Mahajan R, Xing J, Liu SJ, Ly KN, Moorman AC, Rupp L, Xu F, Holmberg SD; for the Chronic Hepatitis Cohort Study (CHeCS) Investigators. Mortality Among Persons in Care With Hepatitis C Virus Infection: The Chronic Hepatitis Cohort Study (CHeCS), 2006-2010. Clin Infect Dis. 2014 Mar 8. [Epub ahead of print]
3 Rein DB1, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011 Jan;43(1):66-72
4 V. Lijewski, et a., Division of Epidemiology and Immunization, Bureau of Infectious Disease, Massachusetts Department of Public Health, “Mortality trends Among People Diagnosed with Hepatitis C Virus Infection: Massachusetts 1992-2009.” Abstract Submitted to the Council of State and Territorial Epidemiologists, fall 2012.
5 Regulations require that both the Initial Preventive Physical Exam (IPPE) and Annual Wellness Visits (AWVs) be performed by qualified health professionals, including physicians and/or other qualified non-physician practitioners. Physician in this context is defined by statute as a doctor of medicine or osteopathy, with no specification related to primary care as would be found in §1883(u)(6), §1833(x)(2)(A)(i)(I) or § 1833(x)(2)(A)(i)(II) of the Social Security Act. (See 42 CFR §§ 410.15-16).
6 Centers for Disease Control and Prevention, Locations and Reasons for Initial Testing for Hepatitis C Infection- Chronic Hepatitis Cohort Study, United States, 2006-2010, MMWR Morb Mortal Wkly Rep. 2013, Aug. 16; 62(32):645-8.
7 Tohme RA, Xing J, Liao Y, Holmberg SD. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010, Am J Public Health 2013; 103:112–9.
8 Lowry, Fran, Babyboomer Hep C Screening Practical in Emergency Rooms, (Mar. 20, 2014), available at: http://www.medscape.com/viewarticle/822310.
9 See e.g. Cisneros GO1, Douaihy AB, Kirisci L. Access to Healthcare Among Injection Drug Users at a Needle Exchange Program in Pittsburgh, PA. J Addict Med. 2009 Jun; 3(2):89.
10 New York Department of Health, NYS Hepatitis C Testing Law Frequently Asked Questions, available at: https://www.health.ny.gov/diseases/communicable/hepatitis/hepatitis_c/rapid_antibody_testing/faqs.htm#quest_3
11 See e.g. 73 FR 69726 (Nov. 19, 2008); 75 FR 73170 (Nov. 29, 2010), and 42 CFR §410.64.
12 See Transmittal 131, “Screening for the Human Immunodeficiency Virus (HIV) Infection,” (Feb, 23, 2011), available at http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R131NCD.pdf.
13 See Transmittal 126, “Counseling to Prevent Tobacco Use,” (Sept. 30, 2010), available at http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R131NCD.pdf
14 For instance, insurers may use “established techniques and the relevant evidence base” to implement “reasonable medical management techniques to determine the frequency, method, treatment, or setting” for a preventive service recommended by the USPSTF, ACIP, or HRSA to the extent not specified by those bodies. This regulation therefore implies that there is not a pre-established setting in which USPSTF guidelines must be implemented. (See 75 FR 41726 (Jul. 19, 2010), 41728-9; 45 CFR §147.130(a)(iv)(B)(4)(4)). Moreover, CMS has not provided any reference to a “relevant evidence base” with respect to its primary care restriction.
References:
1. CDC MMWR/Vol.61/No.4 August 17, 2012
2. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born between 1945-1965. Morbidity and Mortality Recomm Rep 2012; 61:1-1
3. Ly KN, Xing J, Klevens RM et al. The increasing burden of mortality from viral hepatitis in the United States between 1999-2007. Ann Intern Med 2012; 156:271-8
4. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born between 1945-1965. Morbidity and Mortality Recomm Rep 2012; 61:1-1
5. Ferrante JM, Winston DG, Chen PH, de la Torre AN. Family physicians' knowledge and screening of chronic hepatitis and liver cancer. Fam Med 2008;40:345–51; Shehab TM, Sonnad SS, Jeffries M, Gunaratnum N, Lok AS. Current practice patterns of primary care physicians in the management of patients with hepatitis C. Hepatology 1999;30:794–800; Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care
physicians in the USA: results of a national survey. J Viral Hepat 2001;8:377–83.
6. Ghany MG, Strader DB, Thomas DL, Seeff LB, American Association for the Study of Liver D. Diagnosis, management, and treatment of hepatitis C: an update. [Practice Guideline.] Hepatology 2009;49:1335–74; Ghany M, Nelson D, Strader D, Thomas D, Seeff L. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011;54:1433–44.
7. Kallman JB, Arsalla A, Park V, et al. Screening for hepatitis B, C and non-alcoholic fatty liver disease: a survey of community-based physicians. Aliment Pharmacol Ther 2009;29:1019–24.
1 USPSTF Recommendation Statement: Screening for Hepatitis C Infection in Adults. Release date- June 25, 2013. Available online at: http://www.uspreventiveservicestaskforce.org/uspstf12/hepc/hepcfinalrs.htm#ref19
2 2 Coffin, Phillip O, Stevens, Anne M, et al. "Patient acceptance of universal screening for hepatitis C infection." BMC Infectious Diseases. June 2011.
3 Ward JW. The epidemiology of chronic hepatitis C and one-time hepatitis C virus testing of persons born during 1945 to 1965 in the United States. Clinical Liver Dis. 2013; 17:1-11.
4 USPSTF Recommendation Statement: Screening for Hepatitis C Infection in Adults. Release date- June 25, 2013. Available online at: http://www.uspreventiveservicestaskforce.org/uspstf12/hepc/hepcfinalrs.htm#ref19
5 Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-74.
6 European Paediatric Hepatitis C Virus Network. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005; 41(1):45-51.
7 Dienstag JL, McHutchison JG. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006; 130(1):225-30.
8 Centers for Disease Control and Prevention. Recommendations for the prevention and control of hepatitis C (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998; 47(RR-19):1-39.
9 Ward JW. The epidemiology of chronic hepatitis C and one-time hepatitis C virus testing of persons born during 1945 to 1965 in the United States. Clinical Liver Dis. 2013; 17:1-11
10 Centers for Disease Control and Prevention. Recommendations for the prevention and control of hepatitis C (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998; 47(RR-19):1-39.
11 Neff et al. 2011. Gastroenterol Hepatol (N Y). 2011 October; 7(10): 661¡V671
12 Rein, DB, et al. The Cost-Effectiveness of Birth Cohort Hepatitis C Antibody Screening in U.S. Primary Care Settings. American Association for the Study of Liver Diseases. 2011
13 Brillman JC, Crandall CS, Florence CS, et al. Prevalence and Risk Factors Associated with Hepatitis C in ED Patients. Am J Emerg Med. 2002;20(5):476-80.
i Celum, C., Bolan, G., et al. Patients Attending STD Clinics in an Evolving Health Care Environment: Demographics, Insurance Coverage, Preferences for STD Services and STD Morbidity. Sexually Transmitted Diseases. 1997 November. Volume 24, Number10, pp. 599-605.
ii Centers for Disease Control and Prevention, Locations and Reasons for Initial Testing for Hepatitis C Infection- Chronic Hepatitis Cohort Study, United States, 2006-2010, MMWR Morb Mortal Wkly Rep. 2013, Aug. 16; 62(32):645-8.
iii Ibid.
iv Ibid.
v Tohme RA, Xing J, Liao Y, Holmberg SD. Hepatitis C testing, infection, and linkage to care among racial and ethnic minorities in the United States, 2009–2010, Am J Public Health 2013; 103:112–9.
1 Centers for Disease Control and Prevention 9CDC), Fact Sheet accessed online 4/1/2014 at: http://www.cdc.gov/hepatitis/Populations/hiv.htm
2 Aberg, et al, “Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America,” Clin Infect Dis. (2013) doi: 10.1093/cid/cit665 (accessed online 34/1/2014).
References
1Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Ann Intern Med. 2012; 55(8):1047-55.
2Southern WN, Drainoni ML, Smith BD, Christiansen CL, McKee D, Gifford AL, et al. Hepatitis C testing practices and prevalence in a high-risk urban ambulatory care setting. J Viral Hepat. 2011;18:474-81.
3Volk ML, Tocco R, Saini S, Lok AS. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750-5.
4Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17:548-55.
5NACCHO. (2013). Local Health Department Job Losses and Program Cuts: Findings from the 2013 Profile Study. Retrieved March 28, 2014 from http://www.naccho.org/topics/infrastructure/lhdbudget/upload/Survey-Findings-Brief-8-13-13-2.pdf.
Appendix B
We are including the preliminary language for the national coverage determination (NCD) which will be effective immediately. The language is subject to formal revisions and formatting changes prior to the release of the final NCD in the NCD Manual.
Medicare National Coverage Determinations Manual
Chapter 1, Part 4 (Sections 200 – 310.1)
Coverage Determinations
Table of Contents
(Rev.)
210.13 Screening for Hepatitis C Virus (HCV) in Adults
210.13 – Screening for Hepatitis C Virus (HCV) in Adults
(Effective XX XX, 2014)
A. General
HCV is an infection that attacks the liver and leads to inflammation. The infection is often asymptomatic and can go undiagnosed for decades. It is difficult for the human immune system to eliminate the HCV and it is a major cause of chronic liver disease. The presence of HCV in the liver initiates a response from the immune system which in turn causes inflammation. Inflammation over long periods of time (usually decades) can cause scarring, called cirrhosis. A cirrhotic liver fails to perform the normal functions of the liver which leads to liver failure. Cirrhotic livers are more prone to become cancerous and liver failure leads to serious complications, even death. HCV is reported to be the leading cause of chronic hepatitis, cirrhosis and liver cancer and a primary indication for liver transplant in the Western World.
Under §1861(ddd) of the Social Security Act (the Act), the Centers for Medicare & Medicaid Services (CMS) has the authority to add coverage of additional preventive services if certain statutory requirements are met. The regulations provide:
§410.64 Additional preventive services
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Act) that these services are all of the following: (1) reasonable and necessary for the prevention or early detection of illness or disability.(2) recommended with a grade of A or B by the United States Preventive Services Task Force, (3) appropriate for individuals entitled to benefits under Part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
The scope of the national coverage analysis (NCA) for this NCD evaluated the existing evidence and determined if the body of evidence was sufficient for Medicare coverage for screening for HCV in adults at high risk for HCV infection and 1–time screening for HCV infection for adults born between 1945 and 1965, which is recommended with a grade B by the United States Preventive Services Task Force (USPSTF).
B. Nationally Covered Indications
Effective for services performed on or after XX XX, 2014, the CMS has determined the following:
The evidence is adequate to conclude that screening for Hepatitis C Virus (HCV), consistent with the grade B recommendations by the U.S. Preventive Services Task Force (USPSTF), is reasonable and necessary for the prevention or early detection of an illness or disability and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B, as described below.
Therefore, CMS will cover screening for HCV with the appropriate U.S. Food and Drug Administration (FDA) approved/cleared laboratory tests, used consistent with FDA approved labeling and in compliance with the Clinical Laboratory Improvement Act (CLIA) regulations, when ordered by the beneficiary’s primary care physician or practitioner within the context of a primary care setting, and performed by an eligible Medicare provider for these services, for beneficiaries who meet either of the following conditions.
- A screening test is covered for adults at high risk for Hepatitis C Virus infection. “High risk” is defined as persons with a current or past history of illicit injection drug use; and persons who have a history of receiving a blood transfusion prior to 1992. Repeat screening for high risk persons is covered annually only for persons who have had continued illicit injection drug use since the prior negative screening test.
- A single screening test is covered for adults who do not meet the high risk as defined above, but who were born from 1945 through 1965.
The determination of “high risk for HCV” is identified by the primary care physician or practitioner who assesses the patient’s history, which is part of any complete medical history, typically part of an annual wellness
visit and considered in the development of a comprehensive prevention plan. The medical record should be a reflection of the service provided.
For the purposes of this decision memorandum, a primary care setting is defined by the provision of integrated, accessible health care services by clinicians who are accountable for addressing a large majority of personal health care needs, developing a sustained partnership with patients, and practicing in the context of family and community. Emergency departments, inpatient hospital settings, ambulatory surgical centers, independent diagnostic testing facilities, skilled nursing facilities, inpatient rehabilitation facilities, clinics providing a limited focus of health care services, and hospice are examples of settings not considered primary care settings under this definition.
For the purposes of this decision memorandum, a “primary care physician” and “primary care practitioner” will be defined consistent with existing sections of the Social Security Act (§1833(u)(6), §1833(x)(2)(A)(i)(I) and §1833(x)(2)(A)(i)(II)).
§1833(u)
(6) Physician Defined.—For purposes of this paragraph, the term “physician” means a physician described in section 1861(r)(1) and the term “primary care physician” means a physician who is identified in the available data as a general practitioner, family practice practitioner, general internist, or obstetrician or gynecologist.
§1833(x)(2)(A)(i)
(I) is a physician (as described in section 1861(r)(1)) who has a primary specialty designation of family medicine, internal medicine, geriatric medicine, or pediatric medicine; or
(II) is a nurse practitioner, clinical nurse specialist, or physician assistant (as those terms are defined in section 1861(aa)(5));
C. Nationally Non-Covered Indications
Unless specifically covered in this NCD, any other NCD, or in statute, preventive services are non-covered by Medicare.
D. Other
Medicare coinsurance and Part B deductible are waived for these preventive services.
For services provided on an annual basis, this is defined as a 12-month period.
(This NCD last reviewed XX )


