TO: Administrative File: CAG-00442N
FROM: Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group
Joseph Chin, MD, MS
Deputy Director, Coverage and Analysis Group
Lori Ashby, MA
Acting Director, Division of Medical and Surgical Services
Jyme Schafer, MD, MPH
Lead Medical Officer
Michelle Issa, MBA
Lead Analyst
SUBJECT: National Coverage Determination for Screening for Cervical Cancer with Human Papillomavirus (HPV) Testing
DATE: July 09, 2015
I. Decision
The Centers for Medicare & Medicaid Services (CMS) has determined that the evidence is sufficient to add Human Papillomavirus (HPV) testing once every five years as an additional preventive service benefit under the Medicare program for asymptomatic beneficiaries aged 30 to 65 years in conjunction with the Pap smear test. CMS will cover screening for cervical cancer with the appropriate U.S. Food and Drug Administration (FDA) approved/cleared laboratory tests, used consistent with FDA approved labeling and in compliance with the Clinical Laboratory Improvement Act (CLIA) regulations.
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology.
AAFP – American Academy of Family Physicians
ASC-US – Atypical squamous cells of undetermined significance
CC – Conventional cytology
CIN – Cervical intraepithelial neoplasia
CIN2+ - CIN2, CIN3, or cancer
CIN3+ - CIN3 or cancer
CLIA – Clinical Laboratory Improvement Act
FDA – United States Food and Drug Administration
HC2 – Hybrid Capture 2 high-risk HPV DNA test HPV – Human Papillomavirus
HSIL – High-grade squamous intraepithelial lesion
ICC – Invasive Cervical Cancer
LBC – Liquid-based cytology
LSIL – Low-grade squamous intraepithelial lesion
NCI – National Cancer Institute
NIH – National Institutes of Health
OIR – (FDA) Office of In Vitro Diagnostics and Radiologic Health
OUS – Outside of the United States
RCT – Randomized Control Trial
SEER – Surveillance, Epidemiology, and End Results
USPSTF – United States Preventive Services Task Force
CMS initiated this national coverage determination (NCD) to consider coverage under the Medicare Program for cervical cancer screening with a combination of HPV and cytology (Pap smear) testing. The scope of this review was limited to screening for cervical cancer with HPV testing.
Cervical cancer is cancer that forms in tissues of the cervix, which is the organ connecting the uterus and vagina. In the United States it is relatively rare in comparison to other cancers. (NCI/SEER). The natural history of cervical cancer has been well studied and as a result is fairly well characterized. Cancer stage at diagnosis has a strong influence on survival (NCI/SEER):
Percent of Cases and 5-year Relative Survival by Stage at Diagnosis
| Stage |
Percent Cases by Stage |
5-year Relative Survival |
|---|
| Localized | 47% | 90.9% |
| Regional | 36% | 57.4% |
| Distant | 12% | 16.1% |
| Unknown | 4% | 54.4% |
Cervical cancer is almost always caused by a sexually transmitted infection with human papillomavirus (HPV). The HPV viruses also cause other cancers such as anal, vaginal, vulvar, penile, and some oropharyngeal cancers. The HPV virus has over 100 genotypes, of which only approximately fifteen are considered high risk. Of these high risk genotypes, the majority of cervical cancer is caused by 16 and 18. HPV infection is common but only a very small fraction of women infected with HPV will develop cancer. Peak transmission is in young adults. Most of the time HPV infection is cleared by the immune system within one to two years; however, on occasion HPV infection persists. While all factors that determine virus persistence are not known, the main determinant appears to be high risk genotype. When the infection persists it can lead to abnormal cell changes and the precursors of cervical cancer. If precancerous lesions are not treated, they can potentially progress to cancer; however, significant natural regression of even the high grade lesions occurs. Cervical cancer is slow growing most of the time and it can take 10 to 20 years or more for a persistent infection with a high-risk genotype to develop into cancer. Based on SEER data, the median age at diagnosis of ICC is 49 years (NCI/SEER).
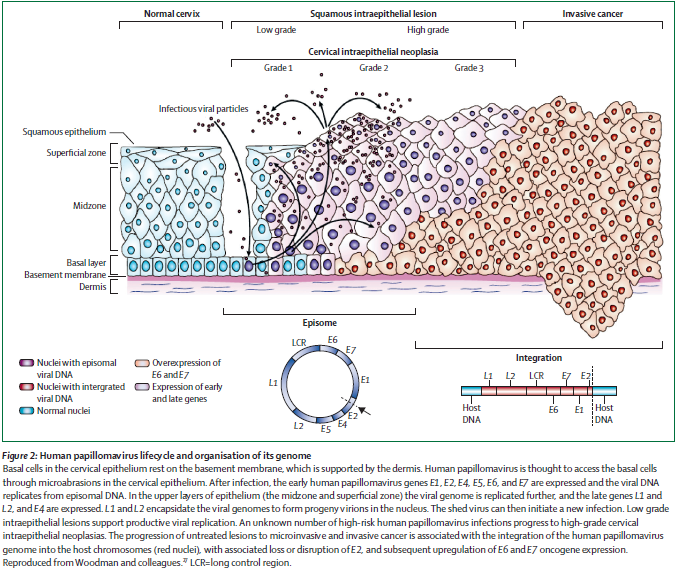
Figure 2: Human papillomavirus lifecycle and organization of its genome. Page 892. Crosbie EJ et al. Human papillomavirus and cervical cancer. Lancet 2013 September 7; 382:889-99.
The aim of cervical cancer screening is detecting precancerous cell abnormalities referred to as cervical intraepithelial neoplasia (CIN) in addition to early cancer. Screening for cervical cancer with a Pap test (cytology), a procedure where cells are scraped from the cervix and examined under a microscope, has contributed to a decline in ICC incidence and mortality. Between 1975 and 2011, the age-adjusted incidence of and mortality rates from cervical cancer in the United States has decreased by more than 50% (SEER Cancer Statistics Review). Despite population based screening, there will be an estimated 12,360 new cases of ICC and 4,020 deaths in the U.S. in 2014. (NCI/NIH/SEER) Estimates are that over 50% of women with ICC have never had screening or have had insufficient screening (Spence et al. 2007). An additional issue is Pap test characteristics. “Data on false-negative results of one-time Pap smears suggest a failure rate of about 28 to 41 percent in developed countries. Imperfect sensitivity as well as errors in sample collection and interpretation across settings underpin the need for frequent repeated screening and underscore interest in developing more accurate, reliable screening tests.” (Vesco et al 2011)
Like the Pap test, the HPV specimen is collected during a pelvic examination and may be subject to sample errors as well. The HPV test is reported as either negative or positive, while the Bethesda System is used for reporting cervical cytology. Biopsy with colposcopy and histologic examination are necessary for diagnosis of CIN or cancer and is considered the “gold standard” for disease assessment. Test thresholds for referral to colposcopy examination can vary. Immediate referral in the United States is generally at a threshold of a cytology result of low-grade squamous intraepithelial lesion (LSIL) (ACOG Guidelines). Abnormal results that do not meet this threshold are generally retested at shorter intervals. Screening strategies can be such that either test is performed alone (primary testing), together (co-testing), or in series as follow-up of abnormal testing (triage). A vaccine has been developed for HPV as a primary prevention strategy.
In 2012, Moyer and colleagues, on behalf of the USPSTF, released updated screening recommendations for cervical cancer: “The USPSTF recommends screening for cervical cancer in women aged 21 to 65 years with cytology (Papanicolaou smear) every 3 years or, for women aged 30 to 65 years who want to lengthen the screening interval, screening with a combination of cytology and HPV testing every 5 years.” (Grade A recommendation) (Moyer for the USPSTF, 2012).
III. History of Medicare Coverage
Sections 1861(s)(14) and 1861(nn) of the Act authorize coverage for screening Pap smear tests for the purpose of early detection of cervical cancer under Medicare Part B. Medicare covers a screening pelvic examination and Pap test for all female beneficiaries at 12 or 24 month intervals, based on specific risk factors. See 42 C.F.R. § 410.56; Medicare National Coverage Determinations Manual, § 210.2. Current Medicare coverage does not include the HPV testing.
Pursuant to §1861(ddd) of the Social Security Act, the Secretary may add coverage of "additional preventive services" if certain statutory requirements are met. Our regulations provide:
42 CFR §410.64 Additional preventive services
(a) Medicare Part B pays for additional preventive services not described in paragraph (1) or (3) of the definition of “preventive services” under §410.2, that identify medical conditions or risk factors for individuals if the Secretary determines through the national coverage determination process (as defined in section 1869(f)(1)(B) of the Social Security Act) that these services are all of the following:
(1) Reasonable and necessary for the prevention or early detection of illness or disability.
(2) Recommended with a grade of A or B by the United States Preventive Services Task Force.
(3) Appropriate for individuals entitled to benefits under Part A or enrolled under Part B.
(b) In making determinations under paragraph (a) of this section regarding the coverage of a new preventive service, the Secretary may conduct an assessment of the relation between predicted outcomes and the expenditures for such services and may take into account the results of such an assessment in making such national coverage determinations.
A. Current Request
CMS received a formal request for a national coverage determination from Jeffery J. Cain, MD, on behalf of the American Academy of Family Physicians (AAFP) to consider coverage of a combination of HPV and cytology (Pap smear) testing for cervical cancer screening. This screening pathway is recommended with a grade A by the USPSTF for females age 30-65 at 5 year intervals as an alternative to triennial Pap smears, which is also recommended with a grade A. The formal request letter can be viewed via the tracking sheet for this NCA on the CMS website at http://www.cms.gov/medicare-coverage-database/details/nca-tracking-sheet.aspx?NCAId=278.
B. Benefit Category
Medicare is a defined benefit program. For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Social Security Act. Since January 1, 2009, CMS is authorized to cover "additional preventive services" if certain statutory requirements are met as provided under §1861(ddd) of the Social Security Act.
IV. Timeline of Recent Activities
| Date |
Action |
| November 25, 2014 |
CMS initiates this national coverage analysis for Screening for Cervical Cancer with Human Papillomavirus (HPV) Testing. A 30-day public comment period begins. |
December 25, 2014 |
First public comment period ends. CMS receives 15 comments. |
April 16, 2015 |
Proposed National Coverage Determination posted. 30-day public comment period begins. |
May 16, 2015 |
Proposed National Coverage Determination 30-day public comment period ends. CMS receives 17 comments. |
V. Food and Drug Administration (FDA) Status
Diagnostic laboratory tests are regulated by the FDA. Several laboratory tests that can detect the presence of high risk human papillomavirus (hr HPV) in cervical specimens, considered a necessary cause of all cervical cancers, are FDA approved and available. The FDA In Vitro Diagnostics database provides specific information on the approved tests for use in HPV co-testing under consideration in this NCA. The two categories of HPV devices include HPV IVD devices that detect, but do not differentiate between different types of HPV (high risk HPV tests) and HPV IVD devices that detect and further differentiate between different HPV types (HPV genotyping tests). Tests to detect HPV are based on recognition of specific HPV DNA or RNA sequences.
A search performed by CMS staff in December 2014 of the FDA’s Office of In Vitro Diagnostics and Radiological Health (OIR) database for HPV testing revealed several approved test devices. These products were approved between 1991 and 2014. The FDA has issued a guidance document for establishing the performance characteristics of in vitro diagnostic devices for the detection or detection and differentiation of human papillomaviruses that are used in conjunction with cervical cytology for cervical cancer screening: Establishing the Performance Characteristics of In Vitro Diagnostic Devices for the Detection or Detection and Differentiation of Human Papillomaviruses.
VI. General Methodological Principles
When making national coverage determinations concerning additional preventive services, CMS applies the statutory criteria in §1861(ddd) of the Social Security Act and regulations at 42 CFR 410.64, and evaluates relevant clinical evidence to determine whether or not the service is reasonable and necessary for the prevention or early detection of illness or disability, is recommended with a grade of A or B by the USPSTF, and is appropriate for individuals entitled to benefits under Part A or enrolled under Part B of the Medicare program.
CMS uses the initial public comments to inform its proposed national coverage determination. CMS responds in detail to the public comments on a proposed national coverage determination when issuing the final national coverage determination. Public comments sometimes cite published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information are redacted or, if the personal health information cannot be successfully redacted, will not be made available to the public.
VII. Evidence
A. Introduction
While a detailed discussion of screening is beyond the scope of this discussion, the basic parameters for screening were established many years ago and are still well accepted to date.
In 1968, Wilson and Jungner reported criteria to consider:
- The condition being screened for should be an important health problem,
- The natural history of the condition should be well understood,
- There should be a detectable early stage,
- Treatment at an early stage should be of more benefit than at a later stage,
- A suitable test should be devised for the early stage,
- The test should be acceptable,
- Intervals for repeating the test should be determined,
- Adequate health service provision should be made for the extra clinical workload resulting from screening,
- The risks, both physical and psychological, should be less than the benefits, and
- The costs should be balanced against the benefits.
(Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. World Health Organization, 1968)
Evaluation of screening tests has been largely standardized in the medical and scientific communities, and the value of a screening test may be assessed according to the following criteria:
- “Simplicity. In many screening programmes more than one test is used to detect one disease, and in a multiphasic programme the individual will be subjected to a number of tests within a short space of time. It is therefore essential that the tests used should be easy to administer and should be capable of use by para-medical and other personnel.
- Acceptability. As screening is in most instances voluntary and a high rate of co-operation is necessary in an efficient screening programme, it is important that tests should be acceptable to the subjects.
- Accuracy. The test should give a true measurement of the attribute under investigation.
- Cost. The expense of screening should be considered in relation to the benefits resulting from the early detection of disease, i.e., the severity of the disease, the advantages of treatment at an early stage and the probability of cure.
- Precision (sometimes called repeatability). The test should give consistent results in repeated trials.
- Sensitivity. This may be defined as the ability of the test to give a positive finding when the individual screened has the disease or abnormality under investigation.
- Specificity. This may be defined as the ability of the test to give a negative finding when the individual screened does not have the disease or abnormality under investigation.”
(Cochrane A and Holland W. Validation of screening procedures. British Medical Bulletin 1971;27(1):3-8. PMID: 5100948).
Health outcomes, benefits, and risks are important considerations. As Cochrane and Holland (1971) further noted, evidence on health outcomes, for example, evidence that screening can alter the natural history of disease in a significant proportion of those screened," is important in the consideration of screening tests since individuals are asymptomatic and "the practitioner initiates screening procedures."
B. United State Preventive Services Task Force (USPSTF)
The USPSTF recommendation for cervical cancer screening (March 2012) states:
- The USPSTF recommends screening for cervical cancer in women age 21 to 65 years with cytology (Pap smear) every 3 years or, for women age 30 to 65 years who want to lengthen the screening interval, screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years.
Grade: A recommendation.
- The USPSTF recommends against screening for cervical cancer with HPV testing, alone or in combination with cytology, in women younger than age 30 years.
Grade: D recommendation.
- The USPSTF recommends against screening for cervical cancer in women younger than age 21 years. Grade: D recommendation.
- The USPSTF recommends against screening for cervical cancer in women older than age 65 years who have had adequate prior screening and are not otherwise at high risk for cervical cancer. Grade: D recommendation.
- The USPSTF recommends against screening for cervical cancer in women who have had a hysterectomy with removal of the cervix and who do not have a history of a high-grade precancerous lesion (cervical intraepithelial neoplasia [CIN] grade 2 or 3) or cervical cancer. Grade: D recommendation.
This recommendation statement applies to women who have a cervix, regardless of sexual history. This recommendation statement does not apply to women who have received a diagnosis of a high-grade precancerous cervical lesion or cervical cancer, women with in utero exposure to diethylstilbestrol, or women who are immunocompromised (such as those who are HIV positive).
The USPSTF assigns one of five letter grades to each of its recommendations (A, B, C, D, I). In July of 2012, the grade definitions were updated to reflect the change in definition of and suggestions for practice for the grade C recommendation.
The following tables from the USPSTF website provide the current grade definitions and descriptions of levels of certainty.
(http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm)
Grade Definitions After July 2012
| Grade |
Definition |
Suggestions for Practice |
| A |
The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
Offer or provide this service.
|
| B |
The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
Offer or provide this service. |
| C |
The USPSTF recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences. There is at least moderate certainty that the net benefit is small. |
Offer or provide this service for selected patients depending on individual circumstances. |
| D |
The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
Discourage the use of this service. |
| I Statement |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
Read the clinical considerations section of USPSTF Recommendation Statement. If the service is offered, patients should understand the uncertainty about the balance of benefits and harms. |
| Level of Certainty |
Description |
High |
The available evidence usually includes consistent results from well-designed, well-conducted studies in representative primary care populations. These studies assess the effects of the preventive service on health outcomes. This conclusion is therefore unlikely to be strongly affected by the results of future studies. |
| Moderate |
The available evidence is sufficient to determine the effects of the preventive service on health outcomes, but confidence in the estimate is constrained by such factors as:
The number, size, or quality of individual studies.
Inconsistency of findings across individual studies.
Limited generalizability of findings to routine primary care practice.
Lack of coherence in the chain of evidence.
As more information becomes available, the magnitude or direction of the observed effect could change, and this change may be large enough to alter the conclusion. |
| Low |
The available evidence is insufficient to assess effects on health outcomes. Evidence is insufficient because of:
The limited number or size of studies.
Important flaws in study design or methods.
Inconsistency of findings across individual studies.
Gaps in the chain of evidence.
Findings not generalizable to routine primary care practice.
Lack of information on important health outcomes.
More information may allow estimation of effects on health outcomes.
|
*The USPSTF defines certainty as "likelihood that the USPSTF assessment of the net benefit of a preventive service is correct." The net benefit is defined as benefit minus harm of the preventive service as implemented in a general, primary care population. The USPSTF assigns a certainty level based on the nature of the overall evidence available to assess the net benefit of a preventive service.
C. Literature Search
CMS searched PubMed from November 2014 to December 2014. We searched for and considered articles in peer reviewed journals and guidelines from May 2011 (publication date of the USPSTF review) to December 2014. General keywords included HPV, screening, co-testing and cervical cancer. Publications that presented original data on screening were considered. Abstracts, animal studies and non-English language publications were excluded. The study was excluded if the sole intent of the study was to examine HPV as a primary screening tool, use as triage, or outcome after treatment.
D. Discussion of Evidence
Question 1: Is the evidence sufficient to determine that screening for cervical cancer with HPV and cytology co-testing is recommended with a grade of A or B by the United States Preventive Services Task Force?
Question 2: Is the evidence sufficient to determine that screening for cervical cancer with HPV and cytology co-testing is reasonable and necessary for prevention or early detection of illness or disability in Medicare beneficiaries?
Question 3: Is the evidence sufficient to determine that cervical cancer screening using HPV and cytology co-testing is appropriate for Medicare beneficiaries?
1. External Technology Assessments
Vesco KK, Whitlock EP, Eder M, Lin J, Burda BU, Senger CA, Holmes RS, Ru R, Zuber S. Screening for Cervical Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 86. AHRQ Publication No. 11-05156-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; May 2011.
Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Inter Med. 15 November 2011; 155:687-697.
Vesco KK, Whitlock EP, Eder M, Burda BU, Senger CA, Lutz K. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the U.S. Preventive Services Task Force. Ann Inter Med 15 November 2011; 155:698-705.
The purpose of the systematic review and companion articles was to assist the USPSTF in updating its 2003 recommendations by focusing on five key questions. Publication dates are from January 2000 through August 2011. Methods of study selection, data extraction, and data synthesis are reported. Quantitative synthesis was not performed due to heterogeneity in the samples, settings, study designs, and instruments.
When to begin screening for cervical cancer
The review supported initiation of screening at age 21 based on a large case-control study of 11,901 women in the United Kingdom, two fair quality cohort studies in the US and United Kingdom, one fair quality study in Iceland, and one good-quality study in the United Kingdom describing the natural history of HPV and CIN. An overall protective effect of screening was demonstrated starting at age 32, which was associated with a 45 percent reduction in the incidence of ICC diagnosis between the ages of 35 and 39. However, earlier screening with an impact on cervical cancer in women aged 25 to 27 could not be completely ruled out. The authors concluded 21 is a reasonable age to begin screening. Screening before this age is complicated by high rates of HPV infection and abnormalities that will regress, with very few cancer cases.
Cytology preparation
Liquid-based cytology (LBC) and conventional cytology (CC) did not differ significantly in sensitivity or specificity for detection of CIN2+ or CIN3+.
Screening Strategies
HPV as stand-alone primary screening or followed by cytology triage
Two fair-quality large European trials (NTCC Phase II, Finnish trial) met inclusion criteria. NTCC Phase II was an RCT of 49,196 women aged 25 to 60 years within the national screening program in Italy. The NTCC Phase II indicated that for women older than 35 years HC2 was about 40% more sensitive but 3-5% less specific than CC for CIN2+ or CIN3+ at a threshold of ASC-US or LSIL referral. The Finnish trial was an RCT within the national screening program of 71,337 women aged 25 to 65 years that compared HPV with cytology triage to CC with a threshold of LSIL+ for referral. In this trial HPV with cytology triage identified about one-third more CIN2+ or CIN3+ than CC. For younger women in NTCC Phase II, cumulative detection of both CIN2+ and CIN3 was doubled in the HPV arm relative to CC with almost no ICC found in either arm. Colposcopy referrals were markedly increased in the HPV primary screening arm compared with CC. Referral patterns were not similar to current U.S. practice. Cumulative colposcopies and cumulative relative positive predictive value over both screening rounds was not reported, limiting conclusions on benefits and burdens of the strategy comparison. In the Finnish trial, HPV with CC triage was not much different than cytology in either CIN3+ detection or immediate colposcopy. Results from the Finnish trial were not complete at the time of this review.
Six diagnostic accuracy studies were also reviewed (37,431 participants). For CIN3+ outcomes, sensitivity ranged from 86% to 97% for HPV testing versus 46% to 50% for cytology at a colposcopy referral threshold of ASC-US. For CIN2+, sensitivity ranged from 63% to 98% for HPV testing versus 38% to 65% for cytology. Specificity for CIN2+ and CIN3+ was 3 to 5 percentage points lower for HPV testing.
The authors state, “Based on large trials, primary screening using a clinically validated HPV test, such as HC2, appears very promising in women aged 35 years and older, particularly when coupled with reflex cytology to triage positive HPV results before colposcopy.”
Combination HPV and cytology as primary screening (co-testing)
Four large fair-quality European RCTs (NTCC Phase I, POBASCAM, Swedescreen, ARTISTIC) compared co-testing to cytology screening alone in 127,149 women aged 20 to 64 years. Cumulative CIN3+ detection was the same in both arms after two rounds of screening in all four RCTs, but the authors state that this may reflect more stringent colposcopy referral protocols compared with the NTCC Phase II primary HPV screening trial. Most co-testing studies did report reduced CIN3+ in the second round of screening compared to cytology. Cumulative ICC was similar or slightly higher in cytology alone compared with co-testing, however there was incomplete reporting of follow-up. Three of four trials had a relatively high threshold for referral (such as HSIL+ after cytology or persistent HPV positive and/or abnormal cytology). None of these three trials had complete reporting for screening round 2. Only NTCC Phase I found a cumulative increase in CIN detection with any CIN measure. In this trial women were referred to colposcopy immediately at a lower threshold. Relative to cytology alone, this strategy increased both CIN2+ and CIN3+ after one screening round and cumulative CIN2+ overall. It did not significantly decrease CIN3+ in round 2 or cumulative CIN3+. ICC was higher in the cytology arm in both rounds but the numbers are small which complicate interpretation. The increase in CIN2+ could be overdiagnosis of disease that may regress. The authors state, “Indirect comparisons between NTCC Phase I and II in older women suggest no additional benefit from co-testing above HPV primary screening alone, but possible increases in false positives.” Cumulative colposcopies were reported in only two trials, both reporting slight increase in the co-testing arm. The authors further state, “More rounds of screening could help determine if there may be other values for co-testing, such as identification of a cohort negative on both tests, that are appropriate for prolonged intervals before rescreening.”
Two co-testing trials included women younger than age 30 or 35 years (NTCC Phase I and ARTISTIC), however complete age-specific results are not available for ARTISTIC. In NTCC Phase I, a study of 11,810 women aged 25 to 34, CIN2+ detection was relatively greater after round 1 and cumulatively with co-testing, which the authors suggest could reflect overdiagnosis of regressive disease. No impact on CIN3+ was found. No cancers were found in the co-testing arm, but three were found in the cytology arm. A much higher rate of colposcopy was found in the co-testing arm in comparison to cytology, consistent with increased false positives detected by HPV testing.
Four diagnostic accuracy studies (21,739 participants) reported the absolute test performance of HPV-cytology co-testing. For screening with either test positive deemed as a co-test positive (17,885 study participants from two studies), HC2 plus cytology was more sensitive but less specific than cytology alone for CIN2+ and CIN3+. This combination did not differ in performance from HC2 alone. For screening with both tests positive deemed as a co-test positive (3,852 study participants from two smaller studies), the specificity was better than HC2 alone but interpretation of the sensitivity was limited by a wide CI.
The authors conclude that given the current trial information, co-testing screening in women 30 years and older is more sensitive for CIN2+ than cytology alone, but may add little to HPV screening alone. The magnitude of increase is uncertain. The authors also note that the increased burden of false-positives with HPV-enhanced screening is critical to understand, particularly in the U.S. given the relatively low incidence of cervical cancer and the established practice of repeated screening for cervical cancer. Further, the true net impact of HPV enhanced screening is difficult to judge as complete information on rates of referral, treatment, and harms are currently not known.
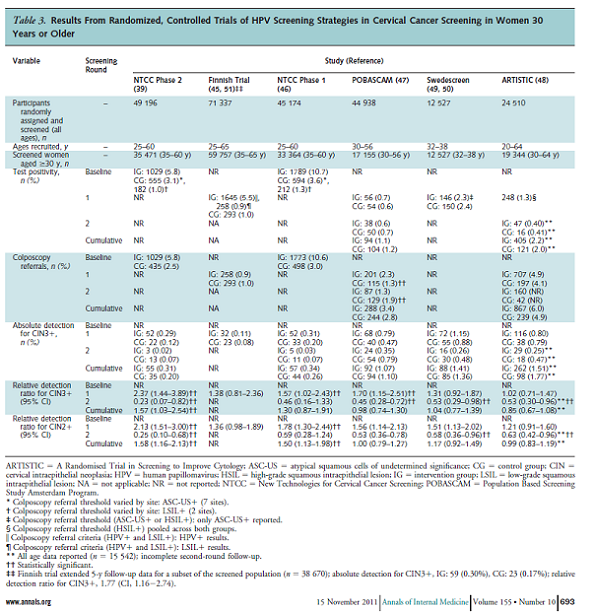
Table 3. Page 693. Whitlock EP, et al. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2011 November 15; 155 (10): 687-697.
HPV for triage of ASC-US or LSIL cytology
Three cross-sectional (two of fair quality and one of good quality) and one prospective cohort study (fair quality) compared HC2 with repeat cytology for triage of women aged 15 to 78 with ASC-US (and LSIL in two studies) cytology results. For ASC-US, pooled estimates for the detection of CIN2+ demonstrated a 12 percent higher relative sensitivity for HC2, with no difference in specificity. HPV testing strategies showed poor specificity for triaging LSIL. For detection of CIN3+ in triage of ASC-US, there was no difference between the two strategies. Two good-quality RCTs evaluated co-testing versus repeat cytology for triage of ASC-US and LSIL. Most with LSIL were found to be HPV positive so this was determined to be an unsuccessful strategy. For ASC-US, there was a nonsignificant increase in CIN3+ detection with co-testing compared to repeat cytology every 6 months for 2 years. Both trials increased colposcopies.
Harms of HPV testing
Four studies examined the psychological impact of HPV testing and found increased levels of anxiety and distress in HPV positive women compared to HPV negative women. At 6 months there were no differences.
HPV tests
There are many methods available to detect HPV. The HPV test most thoroughly studied is HC2 at a positive threshold of 1 pg/ml, and to a lesser extent PCR GP5+/6+. The results in this review reflect these test results. The authors state, “In the absence of adequate RCT data, substitution of other types of HPV testing in cervical cancer screening programs based on these trials should be based on careful consideration of clinical test performance (test positivity, sensitivity, and specificity) when directly compared with HC2, on evidence of test-retest and interlaboratory test reliability, other quality control issues, and cost.”
Limitations
Applicability of trials outside the U.S. to current U.S. practice is complicated by screening and retesting protocols that are generally different. Incomplete reporting of results, including the detection of disease and number of colposcopies, create uncertainty in assessing testing strategies.
Conclusions
- Screening women younger than age 21 does not appear to offer substantial benefit.
- LBC does not appear to differ from CC in test performance.
- The HC2 HPV test is clearly more sensitive for the detection of CIN2+ or CIN3+ compared to cytology alone, but is somewhat less specific with some uncertainty of overdiagnosis of regressive lesions. The true net impact requires confirmation.
- Co-testing in women aged 30 years and older is also more sensitive than cytology alone for the detection of CIN2+ and CIN3+, though the impact on CIN3+ detection is not as clear. There appears to be no additional advantage of co-testing compared to HPV alone.
- HC2 HPV is more sensitive but may be less specific than repeat cytology for the detection of CIN2+ among women with ASC-US. HPV testing is not useful for LSIL or higher grade cytology triage and HPV testing in women younger than age 21 is not advised.
- HC2 is the best studied test in HPV-enhanced screening programs. Substitution of other types of tests should be based on evidence of reliable test performance characteristics in comparison to HC2.
Patanwala IY, Bauer HM, Miyamoto J, Park IU, Huchko MJ, Smith-McCune KK. A systematic review of randomized trials assessing human papillomavirus testing in cervical cancer screening. Am J Obstet Gynecol. 2013 May; 208(5): 343-353. doi:10.1016/j.ajog.2012.11.013.
The purpose of this study was to assess the sensitivity and specificity of HPV testing for cervical cancer screening by summarizing data from randomized trials. Methods, study selection, data abstraction and statistical techniques are reported. Study quality was assessed by using the Quality Assessment of Diagnostic Accuracy (QUADAS) questionnaire. Six studies met these inclusion criteria: 1) the study was a randomized trial comparing HPV-based strategies to cytology-based strategies for primary cervical cancer screening and 2) disease status was determined by colposcopy/biopsy for study participants in whom treatment was warranted. The authors devised a classification system of HPV testing strategies to determine whether differences in outcomes were related to testing strategy:

Figure 1. Page 345. Patanwala I.Y., et al. A systematic review of randomized trials assessing human papillomavirus testing in cervical cancer screening. Am J Obstet Gynecol. 2013 May; 208(5): 343-353. doi:10.1016/j.ajog.2012.11.013.
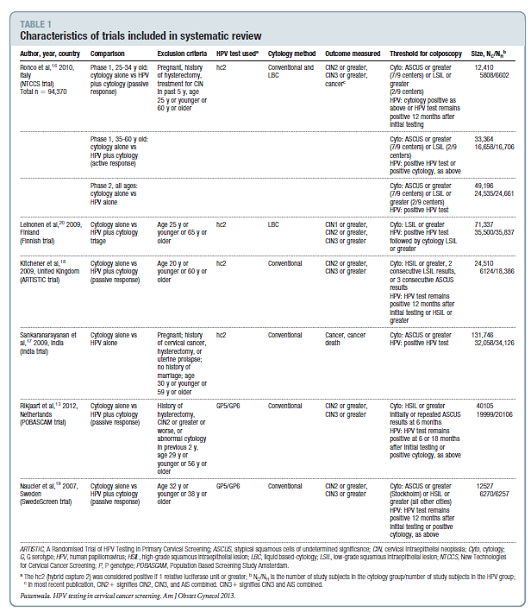
Table 1. Page 347. Patanwala IY, et al. A systematic review of randomized trials assessing human papillomavirus testing in cervical cancer screening. Am J Obstet Gynecol. 2013 May; 208(5): 343-353. doi:10.1016/j.ajog.2012.11.013.
Studies ranged in duration from 6.5 to 8 years and were judged to be of good quality. For each study, the rates of HPV test positivity/abnormal cytology rates or percent referred to colposcopy were either abstracted or calculated. When the positivity/abnormality rate was not recorded, the colposcopy referral rate was used for test positivity. Test positivity ranged from 1.2% to 13.1% for the HPV-based strategies and 1.2% to 7.0% for cytology-based strategies. Relative sensitivity, specificity and positive predictive value were abstracted from the publications or if not stated in the text, relative sensitivity was computed by taking the ratio of disease rate in the HPV based-testing group divided by the disease rate in the cytology-based testing group.
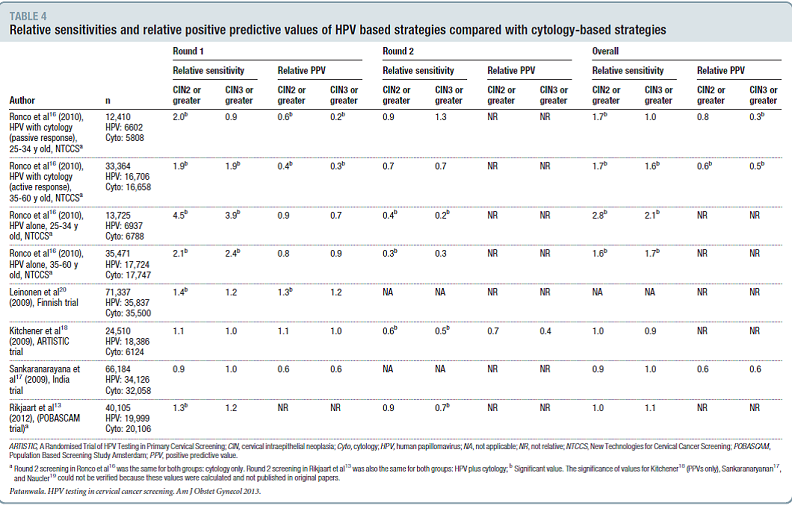
Table 4. Page 350. Patanwala IY, et al. A systematic review of randomized trials assessing human papillomavirus testing in cervical cancer screening. Am J Obstet Gynecol. 2013 May; 208(5): 343-353. doi:10.1016/j.ajog.2012.11.013.
The authors note that in general, HPV testing was more sensitive for CIN2+ and CIN3+ than cytology in the first round of screening and less sensitive in the second round of screening. They also note that higher sensitivities for HPV testing based strategies were observed only for strategies that had immediate referral for a single positive HPV test. As only two studies reported specificities, the relative PPV for HPV based strategies compared with cytology-based strategies was used as a marker for specificity. However, immediate referral for a positive HPV test increases the number of colposcopies needed to detect CIN2+ and CIN3+.
The authors conclude, “In summary, this systematic review indicates that after 2 rounds of screening, HPV-testing based screening strategies are more sensitive than cytology for the detection of CIN3 or greater only when referral to colposcopy follows a single positive HPV test. This strategy results in more colposcopies needed to detect a single case of CIN3 or greater or cancer, especially in women over 35 years of age.”
Bouchard-Fortier G, Hajifathalian K, McKnight MD, Zacharias DG, Gonzalez-Gonzalez LA. Co-testing for detection of high-grade cervical intraepithelial neoplasia and cancer compared with cytology alone: a meta-analysis of randomized controlled trials. J Public Health. 2014 Mar;36(1):46-55. doi: 10.1093/pubmed/fdt057. Epub 2013 Jun 4. PMID: 23735961
The purpose of this meta-analysis of four large RCTs (POBASCAM, ARTSTIC, NTCC Phase I, and Swedescreen) was to compare the screening strategies of co-testing to cytology alone for women ages 21 to 65 for detection of CIN2+, CIN3+, and ICC at follow-up. Results are reported using incidence risk ratios (RR). A random effects model was used to pool RRs. The average age of women ranged from 35.1 to 41 years and length of follow-up ranged from 26 to 92 months. Meta-analyses were performed for the two screening rounds and for the cumulative incidence. At baseline co-testing was associated with a higher CIN2+ detection rate (RR = 1.41, 95% CI: 1.12, 1.76) and a non-significantly higher CIN3+ detection rate (RR=1.15, 95% CI: 0.99, 1.33). Second round co-testing was associated with lower CIN2+ and CIN3+ detection rates (RR = 0.77, 95% CI: 0.63, 0.93; RR + 0.68, 95% CI: 0.55, 0.85). The overall detection rate for both rounds did not differ between co-testing and cytology alone for CIN2+. In the analysis of cumulative detection rate of CIN2+ and baseline CIN2+ heterogeneity was observed among the four trials. There was less heterogeneity in the detection rate of CIN2+ at the second round. Sensitivity analyses were performed to evaluate the exclusion of each trial from the pooled analysis. The authors state, “Following increased detection at baseline screening, a statistically significant 23% reduction in the detection of CIN2+ lesions and a 32% reduction in the detection of CIN3+ lesions was noted at subsequent screening in participants screened with HPV DNA testing plus cytology. These findings suggest that co-testing detected ‘true’ high-grade lesions at baseline, which prompted management according to the pre-specified intervention guidelines in each study and led to reductions in the detection of CIN2+ and CIN3+ at subsequent screening.” The authors conclude that “The results of this meta-analysis suggest that co-testing is an effective strategy for earlier detection of precancerous lesions, which leads to further intervention and decreased rates of detection of high-grade CIN lesions at subsequent screening.”
2. Internal Technology Assessment
Ronco G, Dillner J, Elfstrom K, Tunesi S, Snijders P, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer C, and the International HPV screening working group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomized controlled trials. Lancet 2013 November 3; 383:524-32. http://dx.doi.org/10.1016/S0140-6736(13)62218-7.
The purpose of this study was extended follow-up and data pooling of four European RCTs for estimates of the relative efficacy of HPV-based versus cytology-based screening for prevention of invasive cervical cancer (ICC) in women who have regular screening. All four RCTs were previously reported with precursors of cancer (CIN) as the endpoint in each trial. Endpoint in this study was ICC. The pooled data included 176,464 women aged 20-64 years. Cumulative incidence of ICC was calculated for all women from enrolment to end of observation and for women who were HPV negative at entry in the experimental arm and those who were cytology negative at entry in the control arm. Interventions at first screening round included either liquid-based or conventional cytological testing in the control arm and the experimental arm had either HPV testing alone or co-testing. Interventions at subsequent screening rounds varied in interval based on the country’s organized program (either 3 or 5 years) and also type of screening test (conventional cytology for both arms in two trials, HPV and CC in both arms in one trial, and the primary test corresponding to each arm). Testing between 2.5 years after recruitment and end of follow-up ranged from 70% to 96% but were similar in both arms of each of the four trials. The median follow-up was 6.5 years, during which time 107 ICCs were identified by linkage with registries, review of histological specimens, or from reports. Results are expressed as rate ratios (experimental: HPV based screening / control: cytology based screening) for the incidence of ICC. For all accounted for participants from recruitment to end of follow-up the rate ratio for ICC was 0.60 (95% CI 0.40-0.89). No heterogeneity was found between studies (p=0.52). Any detection of ICC was similar between screening methods during the first 2.5 years of follow-up (0.79, 95% CI 0.46 – 1.36). After 2.5 years, the rate of detection of ICC was significantly lower in the HPV based screening arm (0.45, 95% CI 0.25 – 0.81). The cumulative incidence of invasive cervical carcinoma in women with negative entry tests was 4.6 per 10⁵ (95% CI 1.1 - 12.1) and 8.7 per 10⁵ (95% CI 3.3 - 18.6) at 3.5 and 5.5 years in the experimental arm and 15.4 per 10⁵ (95% CI 7.9 – 27.0) and 36.0 per 10⁵ (95% CI 23.2 – 53.5) in the control arm. The authors concluded different screening protocols used in the four studies did not affect efficacy of HPV testing and that the recorded cumulative incidence of cervical cancer was lower 5.5 years after a negative HPV test than 3.5 years after a negative cytology result. Results were also examined by age at enrollment. The authors conclude that HPV-based screening provides 60-70% greater protection against ICC compared with cytology and that screening with HPV should begin at age 30 and continue with 5 year intervals.
Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, Demuth F, Schiffman M, Wacholder S, Castle PE. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-72.
This 2003-09 observational study reports on 315,061 women aged 30 years and older who enrolled in co-testing at Kaiser Permanente Northern California (KPNC). Their aim with this practice-based study was to: 1) assess the safety of 3-year screening intervals for women who test negative for HPV and have normal cytology and 2) assess if co-testing could identify women at high risk of CIN3+ or ICC over 5 years. The analysis estimated cumulative incidence (includes prevalence at enrolment and incidence after enrolment) of CIN2+, CIN3+, and ICC for each possible combination of co-test results.

Table 1. Page 665. Distribution of worst histological diagnosis by enrolment HPV test and Pap smear. Katki HA, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-72.
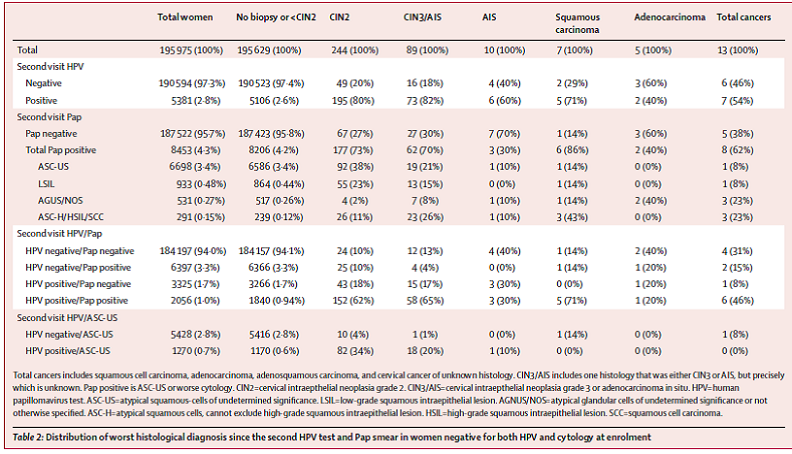
Table 2. Page 668. Distribution of worst histological diagnosis since the second HPV test and Pap smear in women negative for both HPV and cytology at enrollment. Katki HA, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663-72.
The cumulative incidence of ICC was as follows: for women with an HPV negative test, the 5- year cumulative incidence of cancer was 3.8 per 100,000 women per year; for women with a negative HPV test and normal Pap test, the 5-year cumulative incidence was 3.2 per 100,000 women; and for women who were negative by Pap testing the cumulative incidence was 7.5 per 100,000 women. Abnormal cytology increased the cumulative incidence of CIN3+ over 5 years for those with an HPV positive test (12.1% vs 5.9%) but did not substantially increase the risk over 5 years for women with an HPV negative test (0.86% vs 0.16%). Seventy-three percent of the women positive by HPV testing had no cytological abnormality, but comprised 35% of the total women with CIN3+, 29% of the total for ICC, and 63% of the total for adenocarcinomas. The authors conclude, “In summary, our findings show that adding HPV testing to cytology screening promoted earlier identification of the women at high risk of cervical cancer (especially adenocarcinoma) and allowed safe 3-year screening intervals for women negative by both HPV and Pap testing that reduced the burden of screening on patients and clinicians. Furthermore, our findings suggest that 5-year screening intervals for women negative by both HPV and Pap testing might be safe and that HPV testing without adjunctive cytology might be sufficiently sensitive for primary cervical cancer screening. The results of co-testing in 330,000 women over 5 years at KPNC definitely show that concurrent HPV testing and cytology can be feasibly implemented in routine clinical practice to provide powerful prevention of cervical cancer (panel).”
3. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
The MEDCAC was not convened on this topic.
4. Evidence-Based Guidelines
Moyer VA; U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 June 19:156(12):880-891.
Moyer, on behalf of the USPSTF, reported updated recommendations on cervical cancer screening based on the review by Vesco, et al. (2011) (see external technology assessments section above) and modeling by Kulasingam et al. (2011) (see modeling section below). “Recommendations: This recommendation statement applies to women who have a cervix, regardless of sexual history. This recommendation statement does not apply to women who have received a diagnosis of a high-grade precancerous cervical lesion or cervical cancer, women with in utero exposure to diethylstilbestrol, or women who are immunocompromised (such as those who are HIV positive).”
“The USPSTF recommends screening for cervical cancer in women aged 21 to 65 years with cytology (Papanicolauou smear) every 3 years or, for women aged 30 to 65 years who want to lengthen the screening interval, screening with a combination of cytology and HPV testing every 5 years.” (A recommendation)
“The USPSTF recommends against screening for cervical cancer with HPV testing, alone or in combination with cytology, in women younger than age 30 years.” (D recommendation)
Clinical Considerations
Screening tests
It is noted that the screening test commonly used in the United States for HPV testing is HC2. Both HC2 and the polymerase chain reaction-based method have been evaluated in effectiveness trials. “Although alternative HPV detection methods are emerging, the clinical comparability and implications of these methods are not completely understood.”
Screening Interval
HPV testing with cytology is not recommended more often than every 5 years. “Women who choose co-testing to increase their screening interval (and potentially decrease testing) should be aware that positive screening results are more likely with HPV-based strategies than with cytology alone and that some women may require prolonged surveillance with additional frequent testing if they have persistently positive HPV results. Because HPV test results may be positive among women who would otherwise be advised to end screening at age 65 years on the basis of previously normal cytology results alone, the likelihood of continued testing may increase with HPV testing.”
Research Needs and Gaps
The authors note that there is limited evidence on the benefits and harms of HPV testing alone as a screening strategy. Ongoing studies are expected to provide evidence that applies to current U.S. practice. Additionally, more research is needed in determining how individual risk factors may be used to tailor screening to prevent overdiagnosis in women at low risk and underdiagnosis in women at high risk of cancer.
Committee on Practice Bulletins – Gynecology. ACOG Practice Bulletin No. 131: Screening for cervical cancer. Obstet Gynecol. 2012;120(5):1222-1238.
The purpose of this document by the American College of Obstetricians and Gynecologists was to provide a review of the best available evidence regarding screening for cervical cancer.
Screening with co-testing
Co-testing should not be performed in women younger than 30 years. For women aged 30-65 years, co-testing with cytology and HPV testing every 5 years is preferred. The increased sensitivity of co-testing allows for greater detection of CIN 3. Co-testing has an advantage of better detection of cervical adenocarcinoma and its precursors. These screening recommendations do not apply to women with cervical cancer and those who have HIV infection, are immunocompromised, or were exposed to diethylstilbestrol in utero. Data are not adequate to recommend HPV testing alone. Because cervical cancer occurs in a median of 15-25 years after HPV infection, screening should be discontinued after age 65 years in women with evidence of adequate negative prior screening results and no history of CIN 2 or higher.
HPV test considerations
Several tests have been approved by the FDA for the detection of cervical HPV DNA. They assess exfoliated cervical cells for the presence of high risk HPV genotypes. It is recommended that the test kits be used according to FDA-approved labeling, be validated, and meet specific criteria for clinical performance. Unapproved tests should not be used.
Saslow D, Solomon D, Lawson H, Killackey M, Kulasingam S, Cain J, Garcia F, Moriary A, Waxman A, Wilbur D, Wentzensen N, Downs L, Spitzer M, Moscicki A, Franco E, Stoler M, Schiffman M, Castle P, Myers E. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. Am J Clin Pathol 2012; 137:516-542.
This guideline update is based on a systematic evidence review, contributions from six working groups, and a symposium sponsored by the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology.
Screening with co-testing
In women aged 21 to 29 years, HPV testing should not be used either as a co-test or stand-alone test. In women aged 30 to 65, screening with co-testing every 5 years is preferred to cytology every 3 years. The addition of HPV testing to cytology results in an increased detection of CIN 3 and adenocarcinoma of the cervix and its precursors. In this age group, HPV testing alone appears promising but current data is insufficient to recommend this approach. Women aged older than 65 years with evidence of adequate negative prior screening and without a history of CIN2+ within the last 20 years should not be screened for cervical cancer with any modality.
HPV test considerations
HPV testing provides greater sensitivity but lower specificity for CIN3+ and CIN2+ and is more reproducible than cytology. The sensitivity for CIN3+ and CIN2+ should be greater than or equal to 90%, and the percentage of women in the general population who are false positive should be less than or equal to established thresholds from well-validated HPV DNA tests (Meijer et. al. 2009, Stoler et al. 2007). For use in general screening, tests should be FDA approved and meet these clinical standards. “Laboratory-developed tests (LDTs), which are currently exempt from regulatory oversight by the FDA, rarely have undergone the necessary evaluation using clinical endpoints of CIN3+ and CIN2+ in properly designed studies. Therefore, we recommend against the use of LDTs for cervical cancer screening.”
5. Professional Society Recommendations/Consensus Statements
American Academy of Family Physicians
Clinical Preventive Service Recommendation
“The AAFP recommends screening for cervical cancer in women age 21 to 65 years with cytology (Pap smear) every 3 years or, for women age 30 to 65 years who want to lengthen the screening interval, screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years. (2012)” (Grade: A recommendations)
http://www.aafp.org/patient-care/clinical-recommendations/all/cervical-cancer.html
6. Modeling
Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive services Task Force. AHRQ publication No. 11-05157-DF-1. Rockville, MD: Agency for Healthcare Research and Quality; May 2011.
The purpose of this decision model was to address age at which to begin screening with cytology, screening intervals, age at which to end screening, and HPV testing strategies. There is limited direct evidence to address these issues, with most of the evidence coming from epidemiology and natural history studies. The previously validated Duke Cervical Cancer model was used for this analysis. This model describes the natural history of HPV infection including the progression to high-grade precursors and cancer, and the impact of screening and treatment on the prevention of disease progression in a cohort of unvaccinated girls followed until either death or age 100 years. Details of model inputs are included in the appendixes. Test estimates of sensitivity and specificity are based on the companion Oregon EPC review. The main outcome chosen by the Task Force was colposcopies per life-year, as a measure they felt represented a reasonable balance between the burden and benefit of screening. In some analyses, burden included retesting (such as rescreening cytology) in addition to colposcopy. Outcomes include false-positive test results, colposcopies performed, cases of CIN2-3, cases of cervical cancer, and cervical cancer deaths. Four strategies based on USPSTF discussion were examined:
- Cytology, with a repeat cytology test for results of ASC-US. Cytology results of ASC-H, LSIL, or HSIL are referred for colposcopy. Women with normal cytology results are assumed to return to routine screening every 1, 2, 3, or 5 years.
- Cytology, with HPV testing for cytology results of ASC-US. Cytology results of ASC-H, LSIL, or HSIL are referred for colposcopy. Women with normal cytology results are assumed to return to routine screening conducted every 1, 2, 3, or 5 years.
- Cytology and HPV. This strategy is recommended for women 30 and older. Cytology result of ASC-H, LSIL, or higher are referred to colposcopy. Women with a cytology result of ASC-US with HPV positive are triaged to colposcopy, and those who test HPV negative have repeat testing in 1 year. For women with both tests negative, they undergo repeat testing in 3 or 5 years.
- HPV followed by cytology if HPV positive. This strategy is recommended for women 30 and older. Women with normal cytology return for repeat screening at 1, 2, 3, or 5 years.
Estimates of test sensitivity and specificity are as noted below:
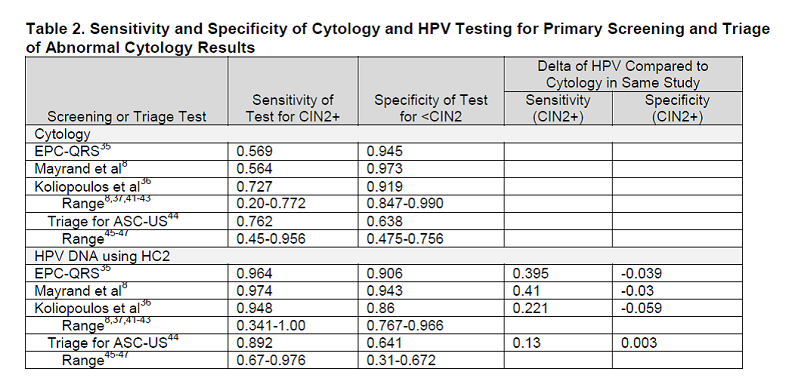
Table 2. Page 8. Sensitivity and specificity of cytology and HPV testing for primary screening and triage of abnormal cytology results. Kulasingam S, et al. Screening for cervical cancer: a decision analysis for the U.S. Preventive Services Task Force. AHRQ publication no. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; May 2011.
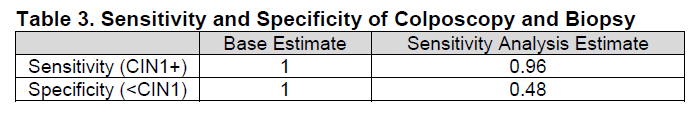
Table 3. Page 8. Sensitivity and specificity of colposcopy and biopsy. . Kulasingam S, et al. Screening for cervical cancer: a decision analysis for the U.S. Preventive Services Task Force. AHRQ publication no. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; May 2011.
Conditional probabilities of cytology results for a given histology result and screening adherence estimates are included in tables 4-6 in the document. Multiple sensitivity analyses were conducted.
Strategies were compared using in units of incremental ratios. For the purposes of the report HPV testing refers only to the use of the Hybrid Capture 2 (HC2) high-risk HPV DNA test (Qiagen Inc., Germantown, MD). At the time of this analysis HPV was only FDA approved as a co-test with cytology.
Screening cytology and age
The analysis of age at which to begin cytology screening shows a high number of false positives and few detected cases of cancer if screening is performed in the teen years. This suggests that disease detected in the teens may be likely to regress and that overdiagnosis and treatment of these lesions may be a concern. The strategy of beginning cytology screening at age 21, and then every 3 years, represents a reasonable balance between the burden and benefit of screening.
Model estimates of women who have been screened with cytology every 3 years prior to age 65 show high burden with small benefit (less than 1 day’s gain in life expectancy per woman) for further screening over age 65. The findings are robust across sensitivity analyses. For women never screened prior to age 65, strategies associated with cytology screening every 2 to 5 years and ending in the 70s represent a reasonable balance between harms and benefits.
Screening with co-testing
Analyses comparing screening cytology with and without HPV testing show that co-testing is an efficient strategy but depends on how the burden is quantified. If colposcopies per life-year is used as the outcome, co-testing strategies are identified as efficient. If screening and triage tests are used to quantify burden, the cytology-only strategies are identified as more efficient than co-testing strategies. In sensitivity analyses, a strategy of HPV testing followed by cytology for high-risk HPV positive women is consistently identified as efficient regardless of which definition of burden is used. However, HPV testing was not yet FDA approved for primary screening at the time of this report.
Addendum to original report
An addendum to the original report presents model analyses on a strategy of screening with cytology every 3 years before age 30 years and then after age 30 years, co-testing every 5 years to age 65. This strategy dominated the scheme of cytology every 3 years, based on a comparison of colposcopies and cancer, using estimates from 2 studies reviewed in the Oregon EPC review. The assumption includes women under age 30 screened with cytology only, beginning at age 21. Sensitivity analyses show that co-testing strategies at different intervals that are identified as efficient depend on the definition used for burden. Additional analyses show primary HPV testing followed by cytology for those who test HPV positive dominates co-testing regardless of either burden definition.
The outcome table for varying co-testing every 3 to 5 years with the test characteristics from Vesco et al. are shown below. Multiple sensitivity analyses were performed which included varying multiple natural history parameters, test characteristics, and screening adherence.
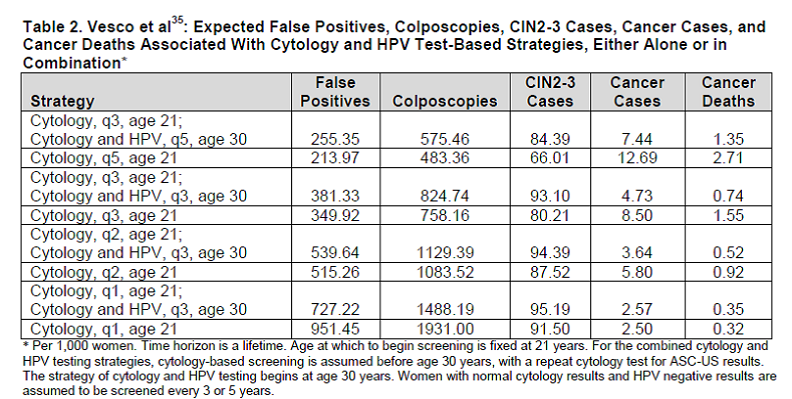
Table 2 Addendum. Page 56. Kulasingam S, et al. Screening for cervical cancer: a decision analysis for the U.S. Preventive Services Task Force. AHRQ publication no. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; May 2011.
Limitations
The authors suggest that a limitation of the analysis is the metric of screening burden. Defining it different ways changes the analysis and affects the efficiency. Also, the choice of strategies using this metric is not consistent with the traditional metric of cost per life-year or cost per quality-adjusted life year. The authors state there are known differences in costs that were not captured in this analysis and therefore results should be cautiously interpreted. Another limitation relates to the low prevalence of disease:
“Other potential limitations are the presentation of results per 1,000 women, and the small differences in disease outcomes which may well be within the margin of error associated with the different parameters used in the model. The USPSTF requested that the results be presented per 1,000 women for consistency with a previous decision analysis for colorectal cancer, so that comparisons could be made. As noted, however, many of the differences in disease outcomes between the strategies, particularly cancer cases and deaths, are small and differ only when a denominator of 100,000 is used. These small differences translate into very small differences in life expectancy between strategies and underscore the fact that the greatest gains from screening will always be from screening unscreened or underscreened women.”
7. Public Comments
During the initial 30-day public comment period (11/25/2014 – 12/25/2014), CMS received 15 comments from various entities including providers, advocacy organizations, trade associations, and the general public. The comments received during the initial 30-day public comment period can be viewed in their entirety on the CMS Website at: http://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=278.
Public Comments on Proposed Decision Memorandum
CMS received 17 comments on the proposed NCD and decision memorandum for screening for cervical cancer with HPV testing. The commenters identified themselves as 3 cytotechnologists or pathologists, 3 other health care providers including physicians and physician assistants, 1 professional organization, 1 consulting group, 1 advocacy group, 1 industry group, 1 insurance company, and 6 from the general public. While all of the commenters generally supported coverage of screening for cervical cancer with HPV testing, some disagreed with the screening interval and others wanted the testing expanded to populations not addressed in the USPSTF recommendation.
Screening Interval
Comment: Several commenters requested that CMS decrease the proposed screening interval from 5 years to 3 years. The commenters cite 3 published articles as evidence.
Response: Based on our analysis of the published clinical evidence and the USPSTF recommendations as well as support from professional organization guidelines, we have determined that the evidence is sufficient to conclude that cervical cancer screening using HPV and cytology co-testing for asymptomatic women every 5 years from ages 30 to 65 is a reasonable balance between benefits and harms and is appropriate for Medicare beneficiaries. We did consider the published articles submitted with the public comments. Gage et al. is a study evaluating primary HPV testing every 3 years in comparison to primary Pap testing every 3 years and cotesting every 5 years. Blatt et al. concludes that cotesting is more sensitive in detection of as compared to primary HPV testing. Commenters also cite a commentary article that expresses an opinion but does not present new data. The three articles do not present new evidence to support a cotesting interval of 3 years. Thus, we are not revising the final decision to reduce the screening interval.
Minimum and Maximum Screening Age
Comment: Several commenters requested that CMS change the proposed minimum and maximum screening age for cervical cancer with HPV testing to include those younger than 30 and older than 65.
Response: The published clinical evidence for cotesting recommends that screening with HPV testing should begin at age 30 as HPV detected below this age would yield an increased number of false positives with little additional benefit to the current recommendation of every 3 year cytology beginning at age 21. Screening with cytology and HPV cotesting in women over age 65 who have been previously routinely screened has shown little benefit with high burden and is not recommended by the USPSTF, however the current age limit applies only to cotesting.
In addition to the screening tests addressed in this NCD, Medicare may cover additional diagnostic testing under the existing regulations at 42 C.F.R. §410.32 if a patient has signs or symptoms of disease.
Other General Issues
Comment: One commenter requested that cotesting screening be expanded to include those exposed to diethylstilbestrol (DES) in utero and those women who have been inadequately screened.
Response: For high risk women, Medicare currently covers screening Pap smear and pelvic examination including clinical breast examination for women at high risk of developing cervical cancer more frequently than every 2 years, which is a shorter time interval than for those at average risk. Women who have not been adequately screened are encouraged to discuss with their physicians and, as recommended, to participate in regular screening with cytology or contesting according to the specific age criterion. This NCD addresses asymptomatic women. Women who have a high-grade precancerous cervical lesion or cervical cancer or had in utero exposure to diethylstilbestrol (DES) are outside the scope of this NCD.
Comment: One commenter requested that Medicare cover annual gynecologic exams.
Response: Medicare currently covers screening Pap tests and pelvic exams to check for cervical and vaginal cancer. These screening tests are covered once every 24 months for all women; and once every 12 months for women at high risk for cervical or vaginal cancer, or women of childbearing age who have had an abnormal Pap test in the past 36 months. Screening mammography is also covered on a routine basis. Medicare also covers a yearly preventive visit called the Annual Wellness Visit that assesses risk for various diseases and provides for follow-up on identified problems.
VIII. CMS Analysis
National coverage determinations are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1862(l) of the Act). Among other things, in order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Screening pap smear tests and screening pelvic exams have a benefit category under §1832, §1861((s)(14) and §1861(nn) of the Act. Screening for HPV is not included within § 1861(s)(14) or § 1861(nn). Since January 1, 2009, CMS is authorized to cover "additional preventive services" (see Section III above) if certain statutory requirements are met as provided under § 1861(ddd) of the Social Security Act.
Question 1: Is the evidence sufficient to determine that screening for cervical cancer with HPV and cytology co-testing is recommended with a grade of A or B by the United States Preventive Services Task Force?
Yes. The USPSTF recommends screening for cervical cancer in women age 21 to 65 years with cytology (Pap smear) every 3 years or, for women age 30 to 65 years who want to lengthen the screening interval, screening with a combination of cytology and human papillomavirus (HPV) testing every 5 years.
Grade: A recommendation.
The USPSTF further notes: “This recommendation statement applies to women who have a cervix, regardless of sexual history. This recommendation statement does not apply to women who have received a diagnosis of a high-grade precancerous cervical lesion or cervical cancer, women with in utero exposure to diethylstilbestrol (DES), or women who are immunocompromised (such as those who are HIV positive).”
Question 2: Is the evidence sufficient to determine that cervical cancer screening with HPV and cytology co-testing is reasonable and necessary for prevention or early detection of illness or disability in Medicare beneficiaries?
The effectiveness of cervical cancer screening with the Pap test is attributed with the decline in incidence in countries that have instituted screening programs. Though the U.S. does not have a national screening program as in many industrialized countries, screening is offered on an individual basis. Incident cancer diagnoses have decreased by more than 50% from 1975-2010 and currently represent only 0.7% of all new cancer diagnoses and 0.7% of all cancer deaths in the U.S. (SEER/NCI/NIH 2014). This low disease prevalence could potentially be lower with a more sensitive test and more straightforward test interpretation. Additionally, screening frequency for women who test negative could be increased if a high negative predictive value is found to be protective over an increased timeframe. “Data on false-negative results of one-time Pap smears suggest a failure rate of about 28 to 41 percent in developed countries. Imperfect sensitivity as well as errors in sample collection and interpretation across settings underpin the need for frequent repeated screening and underscore interest in developing more accurate, reliable screening tests.” (Vesco et al 2011) Further reduction in cancer incidence and mortality could potentially occur if 60% of those currently diagnosed with cancer had been adequately screened.
The etiologic agent in almost all cervical cancers has been identified as HPV. While HPV is the most prominent risk factor for ICC, most HPV infections result in no harm. Accurate and reliable detection of this causal agent would appear to encompass most women who are at risk for ICC though most with infection will never progress to disease. HPV test interpretation is straightforward. Current FDA approved HPV tests detect certain high-risk HPV variants that include most of the subgroups that cause cervical cancer. In contrast, cytology interpretation is relatively subjective. Most recent prevention research has focused on reductions in cancer precursor lesions. Evidence from a treated versus not-treated cohort suggested that over 30 years, approximately one third of precursor cases will advance to ICC, so not all cytology identified as precursors will progress to disease (McCredie 2008). When testing a symptomless population in advance of potential disease, testing and management schemes and disease prevalence influence the estimation of screening benefit and harms. Potential testing schemes could begin with HPV as a stand-alone screening test, as this agent is necessary but not sufficient for disease. Positive tests could then be triaged to cytology or triaged directly to colposcopy with the latter resulting in a high net sensitivity but low net specificity. The focus of this review is HPV and cytology co-testing, performed at the same time. Results of co-testing could be evaluated independently, with a positive HPV test leading to colposcopy, as was done in some European trials. An alternate scheme is cytology initially with HPV testing for any abnormal results. There are a variety of potential screening and management strategies. Net sensitivity and specificity could be somewhat different for each scheme. Sequential testing generally leads to reduced sensitivity with increased specificity whereas simultaneous testing leads to increased sensitivity and reduced specificity. Additionally, predictive value, the probability that a person either has or doesn’t have the disease based on a test result, is related to disease prevalence in the population. Low prevalence of disease generally means a lower predictive value. Accurate quantification of burden and benefit for patient and provider decision making is dependent on all of these variables.
Whitlock et al. reviewed six diagnostic accuracy studies (37,431 participants) comparing HPV screening alone to cytology alone and four diagnostic accuracy studies (21,739) reporting the absolute test performance of co-testing for the USPSTF. For CIN3+ outcomes, sensitivity ranged from 86% to 97% for HPV testing alone versus 46% to 50% for cytology alone at a colposcopy referral threshold of ASC-US. For CIN2+, sensitivity ranged from 63% to 98% for HPV testing alone versus 38% to 65% for cytology alone. Specificity for CIN2+ and CIN3+ was 3 to 5 percentage points lower for HPV testing alone compared to cytology alone. Two studies reporting co-testing results for the detection of CIN2+/CIN3+ , with either test as a positive. This scheme was more sensitive but less specific than cytology alone but did not differ in performance from the HPV test alone. Two studies used a threshold of positive only if both tests were positive unless what they considered to be a high cytology threshold was met. The authors stated wide confidence intervals limited sensitivity comparisons, but this scheme was more specific than HPV alone.
Six large trials randomized HPV based screening strategies to cytology based screening strategies. All are OUS. Early publications report cancer precursors as an endpoint and a later publication with longer follow-up and pooled data reports detected cancer. In all publications, colposcopy was used as a proxy for harms. Two systematic reviews, one meta-analysis, and a review pooling longitudinal trial data combine the results of these various trials. These results should be interpreted with the knowledge that testing methodologies and screening/management schemes were variable across the trials. Protocols used HPV as a stand-alone test or as a cytology co-test and directly referred HPV-positive women to colposcopy or triaged them by cytology. How well one of these testing schemes predicts true disease depends in part on threshold for colposcopy and was different than most U.S. practice. These variations potentially create complexity is estimating benefit and harm for the U.S. population.
- Whitlock et al. included four of the six trials comparing cytology alone to co-testing and reviewed separately trials with primary HPV testing. In contrast to their results on HPV screening alone where it is suggested that HPV increased CIN3+ with a single round (where a positive HPV test led to colposcopy), co-testing did not detect more CIN3+ after 2 rounds than cytology alone. They reported that co-testing detected relatively more CIN2+ and sometimes more CIN3+ after one screening round in comparison to cytology. Slightly lower cases of CIN3+ were detected in the second round of screening and fewer cumulative cancers were reported in the co-testing group. The authors report that interpretation of results in complicated by differences in trial duration, completeness of follow-up, protocol differences, as well as other issues.
- Patanwala et. al. included all six trials. Test positivity ranged from 1.2% to 13.1% for the HPV-based strategies and 1.2% to 7.0% for cytology-based strategies. Table 4 from Patanwala lists the relative sensitivities and relative predictive values for HPV based strategies compared with cytology-based strategies. Interpretation of this table is not straightforward for all of the previously mentioned reasons. As can be seen from the table, relative sensitivities and relative PPV are variable and do not always favor HPV based testing, however more CIN2+ and CIN3+ appear to be detected with HPV-based testing at baseline in comparison to subsequent screening. The authors comment that higher sensitivities for HPV testing-based strategies were observed only for strategies that incorporated immediate referral to colposcopy based on a single positive HPV test.
- Bouchard-Fortier et al. report on the meta-analysis of four studies. Co-testing identified more CIN2+ (statistically significant) and CIN3+ (not statistically significant) as compared to cytology at baseline. In the subsequent round co-testing was associated with a lower detection of CIN2+ and CIN3+. This finding could suggest that co-testing identified more women with true lesions at baseline, reducing lesions at subsequent screening. Based on the absolute difference between total detection rates of CIN2+ lesions, the number of participants needed to screen with co-testing compared with cytology alone is 334 (95% CI: 250, 1000).
- Ronco et al. extended follow-up of four of the RCTs and pooled the data to examine the confirmatory endpoint of ICC rather than previous studies that had cancer precursors as endpoints. For each trial, cumulative incidence of ICC with intentional case ascertainment was calculated for all women from enrollment to end of observation and also for women who were negative at baseline testing. The cumulative incidence of cervical cancer was lower 5.5 years after a negative HPV test than 3.5 years after a negative cytology result. Women with negative test results on entry to the trials had a cumulative incidence of ICC of 4.6 (1.1, 12.1) per 100,000 at 3.5 years and 8.7 (3.3, 18.6) per 100,000 at 5.5 years in the HPV-based testing arm and 15.4 (7.9, 27.0) per 100,000 at 3.5 years and 36.0 (23.2, 53.5) per 100,000 at 5.5 years in the cytology-based testing arm, suggesting an improved negative predictive value. Furthermore, ICC detection rates were similar in both groups up to 2.5 years, which as the authors note could be expected to be prevalent cancers. The rate ratio after 2.5 years was 0.45 (0.25, 0.81), which could reflect efficacy improvement without the prevalent cancers, i.e. reduction in disease incidence. The decreased incidence of ICC for all women from recruitment to end of follow-up was reported as a rate ratio of 0.60 (0.40, 0.89).
The results of the various studies appeared to differ based on inclusion criteria and how results were ultimately analyzed. Taken in totality, these results suggest that screening that included HPV testing detected more CIN2 and CIN3+ at baseline than cytology alone, which led to, at subsequent screenings, decreased rates of detection of CIN3. From the Ronco et al. study, a negative HPV test appeared to confer about a 60% greater chance of protection against ICC (adenocarcinoma and squamous cell). A 50% reduction in disease incidence is suggested with HPV testing. HPV testing is superior to cytology at detecting adenocarcinoma, a cancer type that appears to be increasing. Harms with the current definition of colposcopies and biopsies are difficult to quantify due to incomplete reporting. It is unclear what the addition of co-testing adds to the HPV test alone for primary screening. Further studies will help elucidate optimal screening strategies but the current randomized trial data indicates a reduction in disease with HPV based testing.
Katki et al. provides evidence in routine U.S. clinical practice of the effectiveness over a 5 year timeframe of co-testing. This observational study followed 315,061 women enrolled in a large HMO aged 30 and older to assess the safety of screening interval for women who test HPV negative and have normal cytology and assess if co-testing could better identify 5-year risk. In this population, the cumulative incidence (defined as prevalence at enrolment and incidence after enrolment) of ICC was as follows: for women with an HPV negative test, the 5- year cumulative incidence of cancer was 3.8 per 100,000 women per year; for women with a negative HPV test and normal Pap test, the 5-year cumulative incidence was 3.2 per 100,000 women; and for women who were negative by Pap testing the cumulative incidence was 7.5 per 100,000 women. The authors state that that though the HMO membership is demographically similar to the U.S., it is lacking representation of extremes in income and their risk of cervical cancer has historically been lower for this HMO population than the U.S. national average. HPV testing was significantly better at identifying cervical adenocarcinoma than cytology alone. These practice-based results provide evidence that a negative HPV test provides more reassurance than negative cytology.
Modeling studies for the USPSTF showed similar benefits of co-testing every 5 years and cytology every 3 years, with small differences in expected cancer cases (7.44 versus 8.50, respectively) and cancer deaths (1.35 versus 1.55 deaths, respectively) per 1,000 women.
Colposcopies and biopsies can be used as a proxy for potential harms, though burden could also be defined in other ways such as repeat testing, cost-effectiveness, or some combination. Most analyses have chosen to define burden as colposcopies and biopsies, therefore this metric is the current one for comparison. The potential harms from this procedure include pain, bleeding, and discharge. Published literature includes data indicating the possibility of increased risk for adverse pregnancy outcomes including perinatal mortality, preterm delivery before 34 weeks’ gestation, and low birth weight, though all contentions are somewhat controversial (Arbyn et al. 2008, Naleway et al. 2015, Kyrgiou et al. 2006, Bevis and Biggio 2011). HPV testing has a higher test positive rate than cytology, with rates of HPV positive and normal cytology results ranging from 11% among women aged 30 to 34 years to 2.6% among women aged 60 to 65 years (Datta et al. 2008, Castle et al. 2009, Castle et al. 2011). The higher false positive results with the HPV test raise concerns about unnecessary colposcopies as well as the identification and treatment of precancerous lesions that may regress, greater than that would occur with cytology alone. Using results from the POBASCAM trial, Whitlock et al. note, “On the basis of these findings, 8 CIN2/CIN3 lesions would have to be treated to prevent 1 case of cervical cancer” (Whitlock 2012). As cervical cancer risk and recommended screening vary over a woman’s lifetime, the USPSTF commissioned modeling to reflect lifetime benefit and harm. “Assuming screening with cytology every 3 years before age 30 years and then co-testing every 5 years in a hypothetical cohort of 1000 women, modestly fewer lifetime colposcopies could be expected with co-testing than with cytology (758 vs. 575, respectively), but more lifetime tests could be expected (approximately 5000 more lifetime tests per 1000 women with co-testing)” (Moyer et al. 2012). Harms of cervical cancer screening include anxiety related to positive test results and the time burden of test result follow-up in the case of false positive results. As HPV testing is incorporated more broadly into cervical cancer screening strategies in the U.S., it will be important for patient understanding of risk to better understand the balance
between harms and benefit, particularly considering the low prevalence of cervical cancer.
It is important to select a validated test with regulatory approval (FDA 2011, Stoler 2008). The potential harms of HPV testing are summarized by the FDA in their guidance document:
Failure of devices for the detection, or detection and differentiation, of human papillomaviruses to perform as expected or failure to correctly interpret results may lead to incorrect patient management decisions in cervical cancer screening and treatment. False negative results may lead to delays in the timely diagnosis of cervical cancer and treatment, allowing an undetected condition to worsen and potentially increasing morbidity and mortality. False positive results could lead many women to unnecessarily undergo more frequent screening and potentially invasive procedures such as colposcopy and biopsy. False positive results for the highest risk types of HPV, such as HPV 16 and/or 18, could lead to unnecessarily aggressive treatment of cervical lesions that could impair fertility. Because cervical cancer screening is recommended for virtually all sexually active women and a substantial number of these women will be tested for HPV, the risk scale for potential harm to public health from false negative and false positive HPV results is significant. Therefore, establishing the performance of these devices and understanding the risks that might be associated with the use of these devices is critical to their safe and effective use.
Provider guidelines and recommendations support co-testing at 5 year intervals. Co-testing is the preferred method of screening for several professional groups: the American College of Obstetricians and Gynecologists; the American Cancer Society; the American Society for Colposcopy and Cervical Pathology; and the American Society for Clinical Pathology Screening. HPV testing with validated, reliable test performance characteristics is more reproducible with less subjective analytical characteristics than the Pap smear.
Detection of early stage ICC and treatment leads to excellent 5-year survival, therefore early detection is of great benefit. CIN3+ is an acceptable surrogate for ICC, though some CIN3 will regress. The evidence does suggest that HPV testing detects about 50% more CIN3+ on initial screening than cytology with a reduction in subsequent CIN3+ after HPV testing, meaning that progressive precursors are reduced with HPV testing. Importantly, accrued clinical data suggests a reduction in ICC incidence. HPV testing does lead to more positive tests, including more false positive tests. As detection of early stage ICC can be treated effectively, early detection is effective in mortality reduction. The current evidence is sufficient to determine that cervical cancer screening with HPV and cytology co-testing is reasonable and necessary for prevention or early detection of illness or disability in Medicare beneficiaries. In the future, more data relevant to the U.S. is needed to determine the relative benefits of different screening and management strategies.
Question 3: Is the evidence sufficient to determine that cervical cancer screening using HPV and cytology co-testing is appropriate for Medicare beneficiaries?
HPV is the most common sexually transmitted infection in the world and infection with high-risk HPV is widespread in the United States (Datta 2008). Infection with HPV is the greatest risk factor for ICC (McGraw and Ferrante 2014). Relative population prevalence for each stage in carcinogenesis by age is demonstrated in Figure 2 by Schiffman and Solomon. Infection prevalence peaks soon after the initiation of sexual intercourse. Due to favorable screening criteria including the natural history and epidemiology of cervical cancer and favorable test characteristics, screening asymptomatic women based on age criteria is an effective preventive strategy.
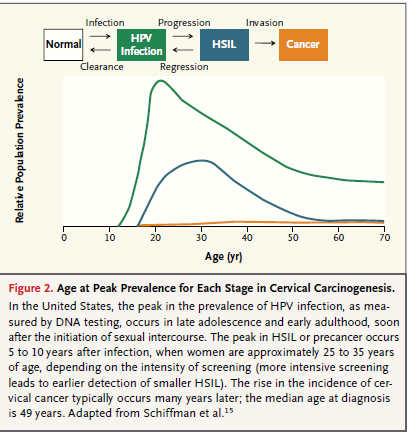
Figure 2. Page 2326. Schiffman M and Solomon D. Cervical-cancer screening with human papillomavirus and cytologic cotesting. New England Journal of Medicine 2013 December; 369:2324-31. DOI:10.1056/NEJMcp1210379.
Age
The evidence for co-testing suggests screening with HPV should begin at age 30 as HPV detected below this age would yield an increased number of false positives with little additional benefit to the current recommendation of every 3 year cytology beginning at age 21. Screening with cytology in women over age 65, in those who have been previously routinely screened, has already shown little benefit with high burden and is not recommended by the USPSTF. In Kulasingam et al, this finding of lack of benefit was robust across model sensitivity analyses. For women over age 65 that have not been previously screened or inadequately screened, the frequency of cytology beyond a baseline test is uncertain.
Frequency of Screening
The evidence for frequency of cervical cancer screening in the USPSTF recommendation is indirect from modeling but it is supported by observational data and follow-up data from the randomized trials. Kulasingam et al. reports on co-testing frequency using a previously validated Duke Cervical Cancer model. The model was previously developed as part of a review of new screening technologies for AHRQ and has realistic assumptions based on current knowledge of cervical cancer, patient and provider behavior, and the natural history of HPV. Analyses including multiple sensitivity analyses with varying parameters were conducted. Model results, longitudinal follow-up of randomized trial data in Ronco et. al., and U.S. based observational data from Katki et al. support the safety of co-testing every 5 years for women who test negative for both tests. For positive test results, risk based management guidelines have been developed though recommendations are not based on randomized trials but rather on observational data or expert opinion. For instance, Schiffman and Solomon present suggested management from data based on cytology testing and co-testing performed by Kaiser Permanente Northern California.
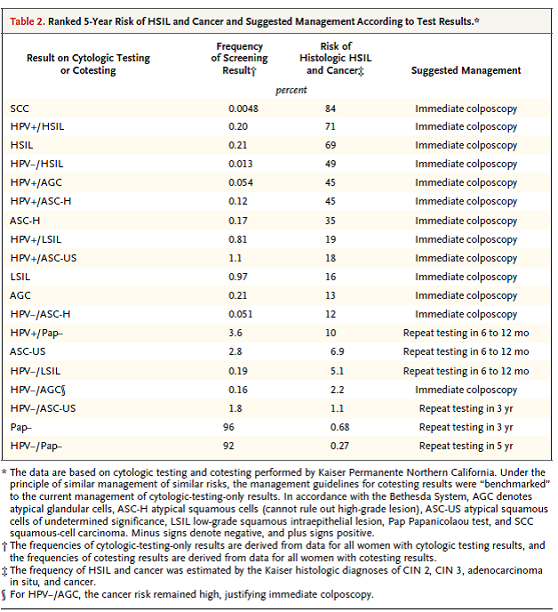
Table 2. Page 2329. Schiffman M and Solomon D. Cervical-cancer screening with human papillomavirus and cytologic cotesting. New England Journal of Medicine 2013 December; 369:2324-31. DOI:10.1056/NEJMcp1210379.
In examination of the screening frequency data and in consideration of disease prevalence and women who are diagnosed with ICC, the greatest gains in reducing incidence and deaths will not be made with a change in screening strategy, but rather from screening those who have not been screened or are underscreened. Including HPV testing safely increases the screening interval and may decrease disease incidence in those who are underscreened.
The evidence is sufficient to determine that cervical cancer screening using HPV and cytology co-testing every 5 years from ages 30 to 65 is appropriate for Medicare beneficiaries. Medicare currently covers the Pap test every 24 months for average risk beneficiaries and every 12 months for high risk beneficiaries. For Pap testing, the current recommendations from various medical societies and USPSTF are that for women who have a cervix, regardless of sexual history, a Pap smear alone every 3 years is a reasonable balance between benefits and harms. This recommendation does not apply to women with increased risk who have received a diagnosis of a high-grade precancerous cervical lesion or cervical cancer, women with in utero exposure to diethylstilbestrol, or women who are immunocompromised. Performing a Pap test more frequently than recommended has not been shown to improve outcomes for women. Cervical cancer has a low prevalence and most diagnoses – 60%- occur in those not previously screened or not screened within 5 years of diagnosis. Overuse of the Pap smear has been well documented.
“For more than a decade, the US Preventive Services Task Force (USPSTF) has recommended that women discontinue Papanicolaou testing if they have received a total hysterectomy and have no history of cervical cancer or if they are older than 65 years and have ongoing and recent normal Papanicolaou test results.5 Nevertheless, in 2010, nearly two-thirds of women reported a Papanicolaou test since their hysterectomy and approximately one-half of women older than 65 years reported a Papanicolaou test in the past 3 years.” (Kepra 2014)
The frequency interval for Medicare coverage of the Pap test is not changed by this determination. To align with the USPSTF recommended frequency, providers are encouraged to perform both tests together every 5 years. We recognize that currently pap smears may be paid more frequently than an HPV test. We believe that a discussion between the beneficiary and the beneficiary's physician is important and permits a careful determination of the appropriateness of specific tests and screening intervals as part of the beneficiary's individual prevention plan.
Disparities
Disparities in cervical cancer screening have been well documented (NCI/NIH, Bernard 2014). Studies have suggested that various factors, including lack of access, economic barriers, and cultural beliefs, are associated with disparities in cervical cancer screening. The Affordable Care Act reduced some economic barriers by eliminating the co-pay for Pap test screening for those who are insured. “There continue to be women who are not screened as recommended, and women who die from this preventable cancer. Evidence-based public health approaches are available to increase women’s access to screening and timely follow-up of abnormal results” (Bernard 2014). Fifty percent of those diagnosed with ICC have not been previously screened, and another 10% were not screened within the five years previous to diagnosis. Great strides can be made if the healthcare community could accomplish screening in this population.
Summary
Incidence and mortality rates for ICC have declined over the past several decades, but ICC is estimated to account for over 4,000 deaths in 2014 (NIH/SEER 2014). Screening with the Pap test combined with effective early treatment have contributed to the observed reduction. Over fifty percent of those diagnosed with cancer have not been previously screened, and another 10% were not screened in the 5 years prior to diagnosis. Accomplishing screening the un-screened or inadequately screened would greatly reduce disease burden. Medicare currently covers frequent Pap test screening. HPV, a necessary but not sufficient causal agent, can be used as a biomarker. Sensitivity for precancers is improved with the addition of HPV testing in comparison to the Pap test alone. However, many precancerous cervical lesions will regress and other detected lesions are indolent and slow-growing and as a result will not become clinically important. Therefore, appreciation of current best scientific knowledge as reflected in USPSTF guidelines maximizes benefit to the patient and reduces the chances of unnecessary testing and treatments. More data is needed on individual risk factors to prevent overdiagnosis and overutilization, and to correct underdiagnosis. Ongoing studies are evaluating HPV for primary, stand-alone screening and an HPV vaccine is available and recommended prior to exposure to HPV. Based on a systematic review of the evidence, we find that HPV and Pap smear co-testing in Medicare beneficiaries using an FDA approved test is reasonable and necessary for the prevention or early detection of illness or disability and appropriate for Medicare beneficiaries.
IX. Conclusion
CMS has determined that the evidence is sufficient to add HPV testing once every five years as an additional preventive service benefit under the Medicare program for asymptomatic beneficiaries aged 30 to 65 years in conjunction with the Pap smear test. CMS will cover screening for cervical cancer with the appropriate U.S. Food and Drug Administration (FDA) approved/cleared laboratory tests, used consistent with FDA approved labeling and in compliance with the Clinical Laboratory Improvement Act (CLIA) regulations.
Appendix A
The following are references submitted in comments as offered by the commenters.
Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015 May;123(5):282-8. doi: 10.1002/cncy.21544.
Gage JC, Schiffman M, Katki HA, et al. Reassurance against future risk of a precancer and cancer conferred by a negative human papillomavirus test. JNCI J Natl Cancer Inst (2014) 106(8): dju153 doi:10.1093/jnci/dju153
Kinney W, Wright T, Dinkelspiel, et al. Current commentary: Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015; 125(2):311-5.Doi:10.1097/AOG.0000000000000632.
Appendix B
This information is representative of Medicare's national coverage determination (NCD) for implementation purposes only. The information is subject to formal revisions and formatting changes prior to the release of the final NCD contractor instructions and publication in the Medicare National Coverage Determinations Manual.
Medicare National Coverage Determinations Manual
Chapter 1, Part 4 (Sections 200 – 310.1)
Coverage Determinations
Table of Contents
(Rev.)
210.2.1 Screening for Cervical Cancer with Human Papillomavirus (HPV) Testing
(Effective XX XX, 2015)
A. General
Medicare covers a screening pelvic examination and Pap test for all female beneficiaries at 12 or 24 month intervals, based on specific risk factors. See 42 C.F.R. § 410.56; Medicare National Coverage Determinations Manual, § 210.2.1 Current Medicare coverage does not include the HPV testing.
Pursuant to §1861(ddd) of the Social Security Act, the Secretary may add coverage of "additional preventive services" if certain statutory requirements are met.
B. Nationally Covered Indications
Effective for services performed on or after XX XX, 2015, CMS has determined that the evidence is sufficient to add Human Papillomavirus (HPV) testing once every five years as an additional preventive service benefit under the Medicare program for asymptomatic beneficiaries aged 30 to 65 years in conjunction with the Pap smear test. CMS will cover screening for cervical cancer with the appropriate U.S. Food and Drug Administration (FDA) approved/cleared laboratory tests, used consistent with FDA approved labeling and in compliance with the Clinical Laboratory Improvement Act (CLIA) regulations.
C. Nationally Non-Covered Indications
Unless specifically covered in this NCD, any other NCD, by statute or regulation, preventive services are non-covered by Medicare.
D. Other
Medicare coinsurance and the Part B deductible are waived for this “additional preventive service.”


