To: File: Electrocardiographic Services (CAG-00158N)
From: Steve E. Phurrough, MD, MPA
Director, Coverage and Analysis Group
Louis B. Jacques, MD
Director, Division of Items and Devices
Stuart Caplan, RN, MAS
Health Insurance Specialist, Division of Items and Devices
Shamiram Feinglass, MD, MPH
Medical Officer, Division of Items and Devices
Subject: Electrocardiographic Services
Date: August 26, 2004
I. Decision
This decision memorandum modifies existing coverage policy by organizing technologies for ambulatory electrocardiography into a framework to aid our contractors in making reasonable and necessary determinations for specific technologies. This decision memorandum does not eliminate coverage of any particular device that is currently covered by existing policy nor does it recommend use of a particular device for particular indications. Further, this decision memorandum does not prescribe a sequence of diagnostic tests for evaluating potential cardiac arrhythmias.
A marketed ambulatory cardiac monitoring device or service is eligible for coverage if it can be categorized according to the framework. Unless there is a specific national coverage determination for that device or service, determination as to whether a device or service that fits into the framework is reasonable and necessary is according to contractor discretion. If a marketed ambulatory cardiac monitoring device or service cannot be categorized according to the framework, then that device is non-covered nationally. CMS, through the NCD process, may create new ambulatory electrocardiographic monitoring device categories if published, peer-reviewed clinical studies demonstrate evidence of improved clinical utility, or equal utility with additional advantage to the patient, as indicated by improved patient management and/or improved health outcomes in the Medicare population (such as superior ability to detect serious or life-threatening arrhythmias) as compared to devices or services in the currently described categories.
II. Background
An electrocardiogram (EKG or ECG) is a graphic representation of electrical activity generated by structures within the heart. Electrodes placed on the body in predetermined locations sense this electrical activity, which is then recorded by various means for review and interpretation. Electrocardiographic recordings are used to diagnose a wide range of heart disease and other conditions that manifest themselves by abnormal cardiac electrical activity known as arrhythmias (or dysrhythmias). Arrhythmias are often classified based on the area of the heart generating aberrant electrical activity; they can range in severity from benign to life-threatening.
For calendar year 2001, CMS allowed charges for ambulatory electrocardiographic monitoring totaled $22.7 million. The most frequent diagnoses associated with these charges were palpitations, followed by syncope and collapse.
Ambulatory electrocardiography (AECG) refers to services rendered in an outpatient setting over a specified period of time, generally while a patient is engaged in daily activities, including sleep. AECG devices are intended to provide the physician with documentation of episodes of arrhythmia, which may not be detected using standard 12-lead EKG. AECG is most typically used to evaluate symptoms that may correlate with intermittent cardiac arrhythmias. Such symptoms include syncope, dizziness, chest pain, palpitations, or shortness of breath. AECG is also used to detect silent myocardial ischemia in patients with documented coronary artery disease. Additionally, AECG is used to determine the efficacy of anti-arrhythmic drug treatment.
Descriptions of ambulatory EKG monitoring technologies
Dynamic electrocardiography devices that continuously record a real-time EKG, commonly known as Holter™ monitors, typically record over a 24-hour period. The long-term recording is captured on magnetic tape or digital medium. The data are then computer-analyzed at a later time, and a physician interprets the computer-generated report.
An "event monitor" (or "event recorder") is a patient-activated or event-activated EKG device that intermittently records cardiac arrhythmic events as they occur. The EKG is recorded on magnetic tape or digital medium.
Cardiac event monitor technology varies among different devices. For patient-activated event monitors, the patient initiates recording when symptoms appear or when instructed to do so by a physician (e.g., following exercise). For self-sensing, automatically triggered monitors, an EKG is automatically recorded when the device detects an arrhythmia, without patient intervention.
Some devices permit a patient to transmit electrocardiographic data transtelephonically (i.e., via telephone) to a receiving center where the data are reviewed. A technician may be available at these centers to review transmitted data 24-hours per day. In some instances, when the EKG is determined to be outside certain pre-set criteria by a technician or other non-physician, a physician is available 24-hours per day to review the transmitted data and to make clinical decisions. These services are known as “24-hour attended monitoring”. In other instances, transmitted electrocardiographic data are reviewed at a later time and are, therefore, considered "non-attended". Cardiac event monitors without transtelephonic capability must be removed from the patient and taken to a location where the electrocardiographic data stored on the device can be reviewed.
Some cardiac event monitoring devices with transtelephonic capabilities require the patient to dial the phone number of an EKG data reception center and to initiate data transmission. Other devices use internet-based in-home computers to capture and store electrocardiographic data. When such devices detect pre-programmed arrhythmias, data are automatically sent via modem and standard telephone lines to a receiving center where the data are reviewed. Internet-based in-home computer systems may also provide the receiving center with a daily computer-generated report that summarizes 24-hours of EKG data.
Certain cardiac event monitors capture electrical activity with a single electrode attached to the skin. Other devices may employ multiple electrodes in order to record more complex EKG tracings. Additionally, devices may be individually programmed to detect patient-specific factors, electrode malfunction, or other factors.
Cardiac event monitors can be further categorized as either "pre-event" or "post-event" recorders, based on their memory capabilities:
- Pre-symptom memory loop recorder. Upon detecting symptoms, the wearer presses a button, which activates the recorder to save (i.e., memorize) an interval of pre-symptom electrocardiographic data along with data during and subsequent to the symptomatic event. Self-sensing recorders (also known as event-activated or automatically triggered) do not require patient input to capture these data. Single or multiple events may be recorded. The device is worn at all times, usually for up to 30 days.
- An implantable or insertable loop recorder (ILR) is another type of pre-symptom memory loop device. These devices are implanted subcutaneously in a patient’s upper chest and may remain implanted for many months. An ILR is used when syncope is thought to be cardiac related, but is too infrequent to be detected by either Holter™ monitor or traditional pre-symptom memory loop recorder.
- Post-symptom recorder. The patient temporarily places this device against the chest when symptoms occur and activates it by pressing a button. This older-style event recorder does not incorporate memory loop technology and telephonically transmits only real-time electrocardiographic data. The device is usually used for up to 30 days.
III. History of Medicare Coverage
Current Medicare National Coverage Determination (NCD) Manual Policy: CMS has a long-standing national coverage policy for electrocardiographic services in §20.15 of the NCD Manual.
CMS has determined that electrocardiographic services fall under the following benefit categories in accordance with the Social Security Act:
- §1861(b)(3), inpatient diagnostic services
- §1861(s)(1), physicians’ services
- §1861(s)(3), outpatient diagnostic services
CMS received inquiries from Carrier Medical Directors and device manufacturers regarding the coverage status of various new electrocardiographic monitoring technologies. No formal requests were submitted to CMS to issue national coverage determinations for specific devices or services. Additionally, §20.15 of the NCD Manual currently discusses payment-related issues that are more appropriately addressed in policy transmittals.
Recognizing the need to address ambulatory electrocardiographic technologies developed since the original publication of §20.15 of the NCD Manual, and to update our national policy, CMS initiated an internal request to reconsider and update §20.15 of the NCD Manual in general. Our review did not address the use of electrocardiographic services for monitoring cardiac pacemakers or implantable cardiac defibrillators, nor did it address AECG for pediatric populations.
The goal of the NCD was to develop a framework of ambulatory electrocardiographic technologies. This framework was created after a review of the relevant evidence and was posted for review and comment. The framework appears in Appendix B.
IV. Timeline of Recent Activities
CMS begins national coverage determination review on January 31, 2003.
V. FDA Status
The ambulatory electrocardiographic devices discussed in this memorandum are cleared for marketing through the Food and Drug Administration 510(k) process as Class II devices for the appropriate indications.
VI. General Methodological Principles of Study Design
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve net health outcomes for patients. The General Methodological Principles of Study Design is located in Appendix A.
VII. Summary of Evidence
CMS is not addressing what is reasonable and necessary with respect to ambulatory electrocardiographic monitoring. Rather, CMS designed the framework categories based on features that may provide clinical advantages for certain patient populations. The framework also allows CMS to categorize existing types of devices and to structure the CMS approach to new technology. The framework appears in Appendix B. The CMS literature search did not identify well-designed clinical studies supporting additional divisions to the framework. The literature search did, however, identify consensus statements on standards of care, collective opinions on the scientific evidence, and expert opinion on the clinical utility of a device category. This Summary of Evidence section highlights some of the more recently published articles that address ambulatory electrocardiographic monitoring. Evidence supporting the framework speaks to the nature of a given heart disturbance or physical symptom, the frequency with which the disturbance or symptom may occur, and the need to intervene based on severity of the disturbance or symptom.
Electrocardiography is the gold standard for noninvasive diagnosis of conduction disturbances and arrhythmias (Kadish et al. 2000). Its use in the ambulatory setting to detect and record cardiac electrical activity during daily activities is widely accepted to aid in clinical decision-making. This use, however, necessitates a framework in which to understand and categorize these devices. Published literature provides the evidence from which CMS created the framework for categorizing electrocardiographic monitoring technologies (see Bibliography).
Kadish et al. (2000) state that there are no specific guidelines to differentiate patients that should undergo continuous monitoring from those that should undergo intermittent monitoring. In general, a continuous recorder, typically used for 24-48 hours, is utilized to evaluate fairly frequent symptoms possibly related to arrhythmias, syncope or near syncope, and recurrent, unexplained palpitations. The authors also state that continuous ambulatory electrocardiographic monitoring is often indicated to evaluate response to antiarrhythmic drug therapy, monitor heart rate in patients with atrial fibrillation, exclude proarrhythmia, analyze patients with pacemakers and implantable cardiac defibrillators, and, though controversial, to assess silent ischemia.
Crawford et al. (1999) state that an intermittent monitoring device is often used for longer periods to evaluate patients with less frequent symptoms. They also state that post-symptom recorders (i.e., those without memory loops) are of limited utility for patients with incapacitating symptoms or loss of consciousness in that the patient may be unable to activate the device immediately following the symptomatic episode. They are best used for infrequent, less serious, sustained symptoms that do not incapacitate the patient.
In an approach to the patient with undiagnosed syncope, Goldschlager et al. (2003) present an evaluation and treatment algorithm that includes using a memory loop recorder in patients with no apparent heart disease on initial evaluation, rather than using a continuous recording device.
Zimetbaum et al. (1998) conducted a prospective cohort study of 105 consecutive outpatients referred for 24-hour attended memory loop recorders for evaluating palpitations. The study measured diagnostic yield, incremental cost, and cost-effectiveness for each week of monitoring. Diagnostic events were considered serious if they included any of the following rhythm disturbances: atrial fibrillation or atrial flutter of any duration, sustained supraventricular tachycardia, symptomatic sustained or non-sustained ventricular tachycardia, junctional rhythm, sinus bradycardia < 50 beats/minute, and complete or high grade heart block. With respect to diagnostic yield for serious arrhythmias, the authors conclude that most new diagnoses are made during the first 2 weeks of monitoring. During week 1, 30 of 105 patients received a new diagnosis of serious arrhythmia. During week 2, 17 received a new diagnosis of serious arrhythmia. During week 3, there was 1 new diagnosis. The authors reported no new diagnoses in week 4.
Krahn et al. (1999) state that despite a wide range of ambulatory electrocardiographic devices, the cause of syncope is not determined after Holter™ evaluation in 38% to 47% of patients. They state that even after tilt table testing, the cause of syncope remains undiagnosed in 10% to 26% of patients. They also reported on using patient-activated implantable memory loop recorders for up to 18 months to evaluate 85 patients with undiagnosed, recurrent syncope. During a mean 10-month follow-up period, 58 patients experienced symptoms. The implantable device detected and recorded an arrhythmia in 42% of patients who activated their recorder during a symptomatic period. Such loop recorder monitoring successfully established a correlation between symptoms and rhythm in 86% of these 58 patients in the group who had recurrent symptoms during the follow-up period.
Ambulatory electrocardiographic monitoring is contraindicated if it results in delayed hospitalization or treatment in patients whose symptoms have an etiology already identified through other diagnostic tests (Kadish et al. 2000). Such monitoring is also not ideal when used as a tool to detect ischemia in patients who are able to undergo exercise testing, or for screening asymptomatic patients for cardiac ischemia.
Public Comment
CMS received comments in support of this framework from the American College of Cardiology (ACC) and the North American Society of Pacing and Electrophysiology (NASPE). These organizations responded that the framework accurately represents the categories of ambulatory electrocardiographic monitoring devices currently in use.
VIII. CMS Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act §1869(f)(1)(B). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be "reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member." §1862(a)(1)(A).
In this case, CMS is not using the NCD process to make a reasonable and necessary determination. Rather, with the assistance of public comment, the NCD creates a framework, presented below, that allows classification of existing electrodiagnostic devices and services into distinct categories.
For example, a device that activates upon sensing an arrhythmia, along with automatically retaining a pre-event EKG tracing in memory, could be covered as a device under the framework as an event-activated, pre-symptom memory loop recorder. Similarly, a device capable of transmitting events, along with a pre-event EKG tracing, to a central location for review by a physician could be a covered device under the framework as an attended, pre-symptom memory loop recorder.
This NCD does not recommend coverage of particular devices or services for particular indications.
Once an electrocardiographic device or service can be classified according to the framework, evaluation as to whether the device or service is reasonable and necessary is then determined either through an NCD or by contractor discretion.
If a newly marketed ambulatory electrocardiographic monitoring device or service cannot be classified according to the framework then it is noncovered nationally. CMS, through the NCD process, may create new ambulatory electrocardiographic monitoring device categories if published, peer-reviewed clinical studies demonstrate evidence of improved clinical utility, or equal utility with additional advantage to the patient, as indicated by improved patient management and/or improved health outcomes in the Medicare population (such as superior ability to detect serious or life-threatening arrhythmias) as compared to devices or services in the currently described categories.
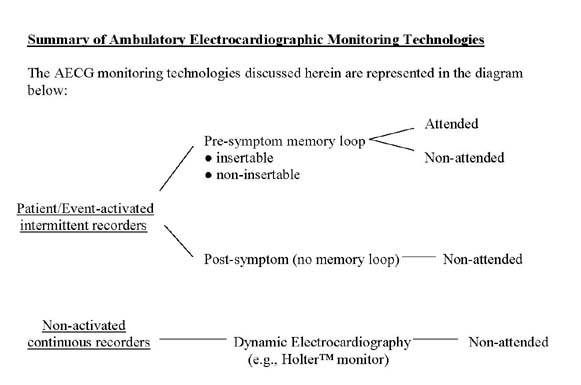
Summary of Ambulatory Electrocardiographic Monitoring Technologies
The AECG monitoring technologies discussed herein are represented in the diagram below:
In conclusion, a marketed ambulatory cardiac monitoring device or service is eligible for coverage if it can be categorized according to the above framework. Unless there is a specific national coverage determination for that device or service, determination as to whether a device or service that fits into the framework is “reasonable and necessary” is according to contractor discretion. If a marketed ambulatory cardiac monitoring device or service cannot be categorized according to the above framework, then that device is non-covered nationally. CMS, through the NCD process, may create new ambulatory electrocardiographic monitoring device categories if published, peer-reviewed clinical studies demonstrate evidence of improved clinical utility, or equal utility with additional advantage to the patient, as indicated by improved patient management and/or improved health outcomes in the Medicare population (such as superior ability to detect serious or life-threatening arrhythmias) as compared to devices or services in the currently described categories.
APPENDIX A
General Methodological Principles of Study Design
(Section VI of the Decision Memorandum)
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
1. Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to which group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is the extent to which differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of which have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
- Randomized controlled trials
- Non-randomized controlled trials
- Prospective cohort studies
- Retrospective case control studies
- Cross-sectional studies
- Surveillance studies (e.g., using registries or surveys)
- Consecutive case series
- Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in which confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation, and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
2. Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to which the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. The goal of our determination process is to assess net health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
3. Assessing the Relative Magnitude of Risks and Benefits
An intervention is not reasonable and necessary if its risks outweigh its benefits. For all determinations, CMS evaluates whether reported benefits translate into improved net health outcomes. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
APPENDIX B
Electrocardiographic Services Framework
Various electrocardiographic diagnostic devices and services are available to physicians. The Centers for Medicare & Medicaid Services (CMS) has created a framework to categorize such devices. CMS, through the NCD process, may create a new device category if published, peer-reviewed clinical studies demonstrate evidence of improved clinical utility, or equal utility with additional advantage to the patient, as compared to device in currently described categories.
Ambulatory electrocardiographic devices are divided into two broad categories. The first category includes non-activated, continuous recording devices. A "non-activated" device does not require a specific trigger or action to capture and record electrocardiographic data. The second category includes patient or event-activated intermittent recording devices. Each of these categories is further described below.
Non-activated, dynamic electrocardiography devices that continuously record a real-time EKG are commonly known as Holter™ monitors. A Holter™ monitor captures and records a real-time, continuous EKG waveform of a patient’s heart rhythm while the patient is engaged in daily activities. The data are stored on magnetic tape or other medium. A physician does not review the gathered electrocardiographic data until after the device is removed from the patient. A Holter™ monitor is generally worn continuously for 24 or 48 hours, during which time the patient may keep a diary of activities and symptoms.
A patient or event-activated intermittent recording device continuously monitors, but does not continuously record, EKG waveforms. The device requires a specific trigger to initiate the recording of electrocardiographic data. Such triggers include, but are not limited to, deliberate action by the patient (usually upon noticing onset of symptoms; "patient activated"), or programmed instructions directing the device to automatically recognize and record specific waveforms or waveform patterns that may or may not produce patient symptoms ("event-activated").
Patient or event-activated intermittent recording devices are designed either with or without a memory loop. The memory loop maintains and stores EKG waveforms for a programmed time interval preceding triggering events. Older event recorders do not contain a memory loop and are unable to record EKG waveforms prior to triggering events. Patient or event-activated intermittent recording devices may transmit stored data to various locations via telephone or other method.
Also, memory loop recorders may be either insertable or non-insertable. (Note: The words insertable and implantable are used interchangeably.) Insertable memory loop recorders are surgically placed under the patient’s skin in the upper chest. Insertable memory loop recorders may remain in place for up to a year, rarely longer, before they are explanted. Non-insertable memory loop recorders are external devices that collect data via electrodes that make contact with a patient’s skin. Non-insertable memory loop recorders are usually used for up to 30 days, sometimes longer.
Memory loop recording devices can be further categorized as to whether or not the recorded EKG data are reviewed by a physician before the device is removed from the patient. When EKG data are reviewed prior to device removal, the recorder is considered “attended”; absent such review, it is considered "non-attended".
Generally, the selection of a particular type of device is based on the frequency with which the patient’s symptoms, thought to be related to cardiac dysrhythmia, occur.



