TO: Administrative File: CAG-00440N
FROM: Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group
Lori Ashby
Acting Director, Division of Medical and Surgical Services
Joseph Chin, MD, MS
Lead Medical Officer
Jamie Hermansen, MPP
Lead Health Policy Analyst
SUBJECT: Proposed Decision Memorandum for Screening for Colorectal Cancer Using Cologuard™ – A Multitarget Stool DNA Test
DATE: 8/11/2014
I. Proposed Decision
The Centers for Medicare & Medicaid Services (CMS) proposes that the evidence is sufficient to cover Cologuard™ – a multitarget stool DNA test – as a colorectal cancer screening test for asymptomatic average risk beneficiaries aged 50 to 85 years.
Therefore, CMS proposes to cover the Cologuard™test once every three years for beneficiaries who meet all of the following criteria:
- Age 50 to 85 years,
- Asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and
- At average risk of developing colorectal cancer (no personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease, including Crohn’s Disease and ulcerative colitis; no family history of colorectal cancers or an adenomatous polyp, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer).
CMS is seeking comments on our proposed decision. We will respond to public comments in a final decision memorandum, as required by §1862(l)(3) of the Social Security Act (the Act).
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology. Additionally, nomenclature for DNA gene markers is used throughout this document. Gene names are expressed in italics.
AA – advanced adenoma
ACG – American College of Gastroenterology
ACS – American Cancer Society
CI – confidence interval
CMS – Centers for Medicare & Medicaid Services
CRC – colorectal cancer
DNA – deoxyribonucleic acid
fDNA – fecal deoxyribonucleic acid
FOBT – fecal occult blood test
FDA – United States Food & Drug Administration
FIT – fecal immunoassay test
HP2020 – Healthy People 2020
MEDCAC – Medicare Evidence Development & Coverage Advisory Committee
NCI – National Cancer Institute
NIH – National Institutes of Health
NNS – number needed to screen
QuARTS – quantitative allele-specific real-time target and signal amplification
sDNA – stool deoxyribonucleic acid
SEER – Surveillance, Epidemiology, and End Results
SSA – sessile serrated adenoma
SSED – Summary of Safety and Effectiveness Data
SSP – sessile serrated polyps
USMSTF – United States Multi-Society Task Force
USPSTF – United States Preventive Services Task Force
CMS initiated this national coverage determination (NCD) to consider coverage for colorectal cancer (CRC) screening using Cologuard™ – a multitarget stool DNA (sDNA) test. We are focusing our review on the commercially available Cologuard™ test since the test evaluates specific DNA markers and fecal hemoglobin using a proprietary analytic algorithm. This proposed decision memorandum does not address the use of stool DNA testing as a diagnostic test to evaluate signs or symptoms of colorectal disease.
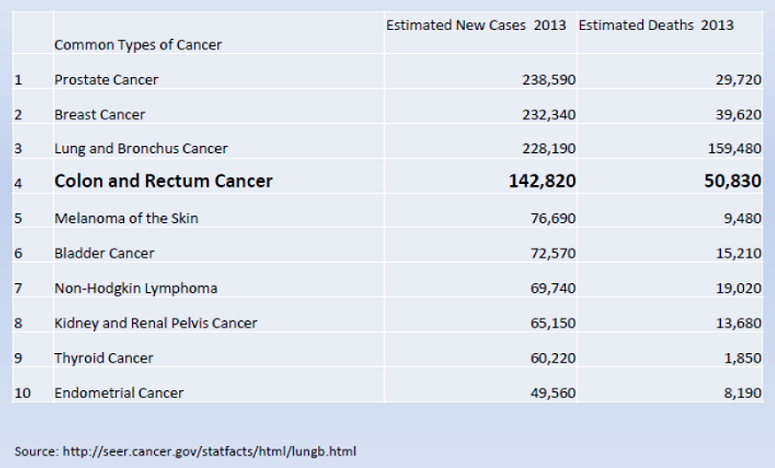
CRC is the fourth most common cancer and the second leading cause of cancer deaths in the United States. It is an important issue for the Medicare population. In 2013, the National Institutes of Health (NIH) National Cancer Institute (NCI) estimated that there will be over 140,000 new cases of colon and rectum cancer in the United States with a median age at diagnosis of 68 years. Overall mortality rates for CRC have declined over the past decade. Primary prevention, early detection and early treatment have contributed to the observed reduction in mortality; however, CRC was estimated to account for over 50,000 deaths in 2013, with a median age at death of 74 years (NIH/SEER 2013).
The natural history of colorectal neoplasia has been well studied.
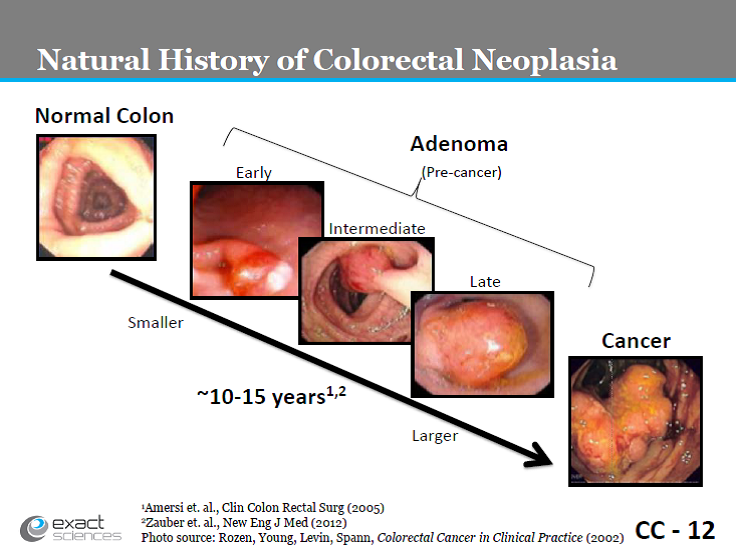
Slide CC-12. Exact Sciences presentation, March 27, 2014.
http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MolecularandClinicalGeneticsPanel/UCM391101.pdf
Early detection of large pre-cancerous adenomas and removal prevents progression of these adenomas to carcinoma, reducing the incidence of colorectal cancer (Mandel, 2000; Nishihara, 2013); while early detection through screening has been shown to improve mortality (Mandel, 1993; Nishihara, 2013; Shaukat, 2013).

http://seer.cancer.gov/statfacts/html/colorect.html
While the screening rate of CRC has increased, there are still missed opportunities as the screening rate in adults aged 50-75 years remains below the Healthy People 2020 goal [Baseline: 52.1 percent of adults aged 50 to 75 years received a colorectal cancer screening in 2008; Target: 70.5 percent (HP2020)].
Stool or fecal DNA testing detects molecular markers of altered DNA that are contained in the cells shed by CRC and pre-malignant colorectal epithelial neoplasia into the lumen of the large bowel. With the discrete natural history of colorectal neoplasia, several events during the development and progression of adenomas to CRC have been identified and classified including: (1) mutational activation of proto-oncogenes (normal genes that are involved in cellular growth and proliferation); (2) mutational inactivation of tumor suppressor genes (normal genes that suppress cellular growth and proliferation); (3) specific chromosomal point mutations; and (4) accumulation of sufficient genetic alterations for tumorigenesis (Vogelstein, 1988; Friend, 1988; Fearon, 1990; Cho, 1992).
DNA markers are released regularly and continuously due to the constant sloughing of the colorectal epithelial cells lining the bowel lumen and end up in the stool stream. Through the use of selective enrichment and amplification techniques, sDNA tests are designed to detect very small amounts of DNA markers to identify CRC or pre-malignant colorectal neoplasia. The Exact Sciences Cologuard™ – multi-target sDNA test is designed to detect three independent sets of markers that exhibit an additive association with CRC and pre-malignant colorectal epithelial neoplasia. The first DNA family targets epigenetic changes (changes in gene expression without DNA sequence change) in the form of gene promoter region methylation. The second DNA family targets specific point mutations. ACTB is a reference gene used for confirmation and quantitative estimation of the total amount of human DNA present in each sample. The third marker is non-DNA based and detects human fecal hemoglobin (FIT test).
Cologuard™ tests for two DNA methylation markers [NDRG4, BMP3], seven point mutations on K-ras [codons 12 and 13], quantitative DNA [β-actin], and fecal hemoglobin. It is a proprietary in vitro diagnostic device that incorporates both sDNA and fecal immunochemical test techniques and is designed to analyze patients’ stool samples for markers associated with the presence of colorectal cancer and pre-malignant colorectal neoplasia (Exact Sciences, 2014).
NDRG4 (N-Myc downstream-regulated gene 4; also known as SMAP-8 and BDM1) is “located at chromosome 16q21 – q22.3, spans 26 kilobases, and contains 17 exons” (protein coding regions) (Zhou, 2001; Melotte 2009). It is “a candidate tumor suppressor gene in colorectal cancer whose expression is frequently inactivated by promoter methylation. NDRG4 promoter methylation is a potential biomarker for the noninvasive detection of colorectal cancer in stool samples” (Melotte, 2009).
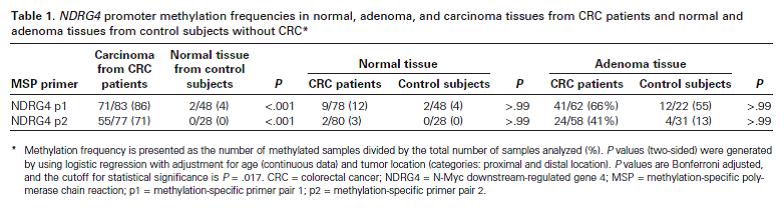
Table 1. Page 920. Melotte et al. N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst. 2009 Jul 1;101(13):916-27.
BMP3 (bone morphogenic protein 3) is “a member of the transforming growth factor beta (TGFB) superfamily” (Loh, 2008) whose methylation has been seen in colorectal polyps and cancers (Zou, 2007; Loh, 2008).
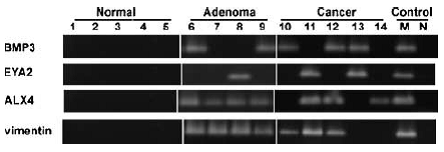
Figure 3. Neoplasm-specific methylation of BMP3, EYA2, ALX4, and vimentin genes. Methylation status was determined by conventional MSP using methylation-specific primers. Representative tissues from normal colon epithelia, adenomas, and cancers. Universally methylated DNA and water were amplified as positive and negative controls, respectively. Page 2689. Zou et al. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol Biomarkers Prev. 2007 Dec;16(12):2686-96.
K-ras (Kirsten rat sarcoma viral oncogene homolog) is located on chromosome 12 and “provides instructions for making a protein called K-Ras that is involved primarily in regulating cell division” (NIH http://ghr.nlm.nih.gov/gene/KRAS). Alterations (activation) of K-ras have been detected in colorectal cancers and adenomas (Bos, 1987; Forrester, 1987; Vogelstein, 1988; Fearon, 1990; Zou, 2007).
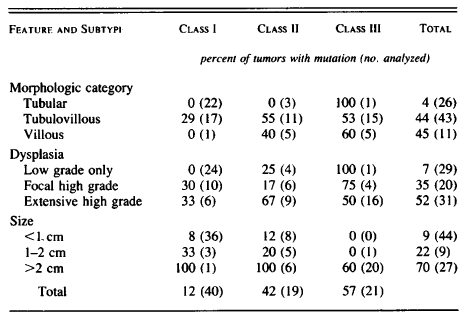
Table 2. Relation between Ras-Gene Mutation and Histopathological Features of Adenomas. Page 528. Vogelstein et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525-32.
The Cologuard™ test functions as a screening tool by generating a single clinically actionable score based on the detection of multiple DNA methylation and mutational markers and fecal hemoglobin, together with an assessment of the total amount of human DNA in each sample. This CRC screening panel of complementary informative markers, and the composite score which is calculated from the analysis of the detection of these markers, increases the likelihood of detection of cancerous or precancerous lesions by a subsequent diagnostic examination (Exact Sciences, 2014).
The multi-target sDNA testing procedure involves optimized sample processing and proprietary assay methodologies for detection of the following informative markers in stool:
- NDRG4 promoter region DNA hyper-methylation;
- BMP3 promoter region DNA hyper-methylation;
- K-ras gene DNA point mutations
- Total human DNA as measured using ACTB (β-actin); and
- Fecal hemoglobin.
As with other noninvasive fecal CRC screening tests (guaiac fecal occult blood tests (FOBT), FIT)), referral of a positive sDNA test to a diagnostic and often therapeutic (if lesions are removed) colonoscopy is essential in the screening framework to reduce incidence of and mortality from CRC.
III. History of Medicare Coverage
Sections 1861(s)(2)(R) and 1861(pp) of the Act authorize coverage for screening colorectal cancer tests under Medicare Part B. Among other things, the statute enables the Secretary to add coverage for “other tests and procedures, and modifications to tests and procedures under this subsection, with such frequency and payment limits, as the Secretary determines appropriate, in consultation with appropriate organizations.” (Section 1861(pp)(1)(D) of the Act; 42 CFR 410.37(a)(1)(v)). Our regulations can be found at 42 CFR 410.37.
In the Physician Fee Schedule Final Rule for 2003, CMS amended the FOBT screening test regulation definition in 42 CFR 410.37(a)(2) to provide that it could include coverage of either (1) a guaiac-based FOBT, or (2) other tests as determined by the Secretary through a national coverage determination (67 Fed. Reg. 79966, 80040) (December 31, 2002). On November 4, 2003, CMS issued a final Decision Memorandum indicating that effective with that date Medicare would cover a screening fecal immunoassay test (FIT) (See Pub. 100-03, Chapter 1, Section 210.3, screening immunoassay FOBT) on an annual basis as an alternative to the guaiac-based FOBT.
In the Physician Fee Schedule Final Rule for 2003, CMS also amended the colorectal cancer screening test regulation in 42 CFR 410.37(a)(1)(v) to provide that in addition to the screening test options already covered under the regulation, it could include coverage of additional colorectal cancer screening tests through issuance of a national coverage determination (67 Fed. Reg. 79966, 80040) (December 31, 2002).
Medicare currently covers the following CRC screening tests for beneficiaries that meet certain frequency and eligibility criteria: (1) screening FOBT, (2) screening flexible sigmoidoscopy, (3) screening colonoscopy, (4) screening barium enema as an alternative to flexible sigmoidoscopy or colonoscopy, and (5) other tests and procedures and modifications to such tests and procedures the Secretary determines appropriate in consultation with appropriate organizations. Since Cologuard™ cannot be classified in any existing category, a new category of CRC screening tests will be created for screening stool or fecal DNA test.
A. Current Request
Exact Sciences Corporation is participating in the FDA – CMS Parallel Review Pilot Program. CMS received a formal request for a national coverage determination from Exact Sciences Corporation, to consider coverage of Cologuard™ – a multi-target sDNA colorectal cancer screening test.
B. Benefit Category
Medicare is a defined benefit program. For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Social Security Act. Congress has specifically authorized coverage of certain screening tests under Part B of the Medicare program. CRC screening tests have a benefit category under §1832, §1861(s)(2)(R) and §1861(pp) of the Act. Specifically, we are using the national coverage determination authority under section 1861(pp)(1)(D) (and implementing regulations at 42 CFR 410.37(a)(1)(v)) to determine whether the scope of the CRC screening benefit should be expanded to include coverage of Cologuard™ – a multitarget stool DNA test.
IV. Timeline of Recent Activities
| Date |
Action |
| 8/11/2014 |
CMS initiates this national coverage analysis for Screening for Colorectal Cancer using Cologuard™ – a multi-target sDNA test and posts the proposed decision memorandum. A 30-day public comment period begins. |
V. Food and Drug Administration (FDA) Status
The Cologuard™ is a FDA-approved test with the following Intended Use and Indications for Use.
Intended Use: Cologuard™ is intended for the qualitative detection of colorectal neoplasia associated DNA markers and for the presence of occult hemoglobin in human stool. Cologuard™ is for use with the Cologuard™ collection kit and the following instruments: BioTek ELx808 Absorbance Microplate Reader; Applied Biosystems® 7500 Fast Dx Real-Time PCR; Hamilton Microlab® STARlet; and the Exact Sciences System Software with Cologuard™ Test Definition.
Indications for Use: Cologuard™ is intended for the qualitative detection of colorectal neoplasia associated DNA markers and for the presence of occult hemoglobin in human stool. A positive result may indicate the presence of colorectal cancer (CRC) or advanced adenoma (AA) and should be followed by diagnostic colonoscopy. Cologuard™ is indicated to screen adults of either sex, 50 years or older, who are at typical average-risk for CRC. Cologuard™ is not a replacement for diagnostic colonoscopy or surveillance colonoscopy in high risk individuals.
VI. General Methodological Principles
When making national coverage decisions concerning the scope of the CRC screening benefit under Medicare Part B, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that a test is suitable and appropriate for general screening in individuals with no signs or symptoms of the condition being screened for in the Medicare population. A detailed account of the methodological principles of study design that the Agency utilizes to assess the relevant literature can be found in Appendix A. In general, features of clinical studies that improve quality and decrease bias include the selection of a clinically relevant cohort, the consistent use of a single good reference standard, the blinding of readers of the index test, and reference test results.
Public comments sometimes cite the published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information (PHI) will be redacted and the PHI will not be made available to the public. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum.
VII. Evidence
A. Introduction
While a detailed discussion of screening is beyond the scope of this discussion, the basic parameters for screening were established many years ago and are still well accepted to date. In 1968, Wilson and Jungner reported criteria to consider:
- The condition being screened for should be an important health problem,
- The natural history of the condition should be well understood,
- There should be a detectable early stage,
- Treatment at an early stage should be of more benefit than at a later stage,
- A suitable test should be devised for the early stage,
- The test should be acceptable,
- Intervals for repeating the test should be determined,
- Adequate health service provision should be made for the extra clinical workload resulting from screening,
- The risks, both physical and psychological, should be less than the benefits, and
- The costs should be balanced against the benefits.
(Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. World Health Organization, 1968)
Evaluation of screening tests has been largely standardized in the medical and scientific communities, and the value of a screening test may be assessed according to the following criteria:
- “Simplicity. In many screening programmes more than one test is used to detect one disease, and in a multiphasic programme the individual will be subjected to a number of tests within a short space of time. It is therefore essential that the tests used should be easy to administer and should be capable of use by para-medical and other personnel.
- Acceptability. As screening is in most instances voluntary and a high rate of co-operation is necessary in an efficient screening programme, it is important that tests should be acceptable to the subjects.
- Accuracy. The test should give a true measurement of the attribute under investigation.
- Cost. The expense of screening should be considered in relation to the benefits resulting from the early detection of disease, i.e., the severity of the disease, the advantages of treatment at an early stage and the probability of cure.
- Precision (sometimes called repeatability). The test should give consistent results in repeated trials.
- Sensitivity. This may be defined as the ability of the test to give a positive finding when the individual screened has the disease or abnormality under investigation.
- Specificity. This may be defined as the ability of the test to give a negative finding when the individual screened does not have the disease or abnormality under investigation.”
(Cochrane A and Holland W. Validation of screening procedures. British Medical Bulletin 1971;27(1):3-8. PMID: 5100948).
Health outcomes, benefits, and risks are important considerations. As Cochrane and Holland (1971) further noted, evidence on health outcomes, for example, evidence that screening can alter the natural history of disease in a significant proportion of those screened," is important in the consideration of screening tests since individuals are asymptomatic and "the practitioner initiates screening procedures." Since a number of colorectal cancer screening tests are available and covered by Medicare, how a new test should be used and how it fits into current recommendations for screening should also be considered.
B. Literature Search
CMS searched PubMed from 2008 to March 2014. General keywords included stool/fecal DNA and colorectal cancer. Publications that presented original data on screening with DNA testing were considered. CMS typically considers a category of tests or devices, rarely making decisions on brand specific items; however, since the Cologuard™ test evaluates specific DNA markers and hemoglobin using a proprietary analytic algorithm, we focused our review on the recently FDA-approved, commercially available Cologuard™ test. Since PreGen-Plus™ was the predecessor test produced by the same manufacturer, publications on this test were also reviewed. By including the prior test, advancements in technology, marker selection and analytics may be shown in the process of developing and marketing an improved screening sDNA test. Abstracts, animal studies and non-English publications were excluded.
C. Discussion of Evidence
Question 1: Is the evidence sufficient to determine that the Cologuard™ test is a suitable colorectal cancer screening test for prevention or early detection in Medicare beneficiaries?
Question 2: Is the evidence sufficient to determine that colorectal cancer screening using the Cologuard™ test is appropriate for Medicare beneficiaries?
1. External Technology Assessments
None were located.
2. Internal Technology Assessment
Ahlquist DA, Sargent DJ, Loprinzi CL, Levin TR, Rex DK, Ahnen DJ, Knigge K, Lance MP, Burgart LJ, Hamilton SR, Allison JE, Lawson MJ, Devens ME, Harrington JJ, Hillman SL. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008 Oct 7;149(7):441-50, W81.
Ahlquist and colleagues reported the results of a cross-sectional study “to compare the precommercial stool DNA test (SDT-1), which was studied by Imperiale and colleagues (21), with widely used fecal occult blood tests for the detection of screen-relevant neoplasia, defined as curable-stage colorectal cancer (no distant metastases), high-grade dysplasia, or adenomas larger than 1 cm” and “to explore neoplasm detection by another stool DNA test (SDT-2), which uses a more broadly informative marker panel.” From 2001 to 2007, 4482 “asymptomatic persons age 50 to 80 years who were at average risk for colorectal cancer from communities surrounding 22 participating academic and regional health care systems through direct mail and multimedia advertisements” were enrolled. Exclusion criteria were structural colorectal evaluation (endoscopic or radiographic) within 10 years; fecal blood testing within one year; overt rectal bleeding within one month; previous colorectal resection; aerodigestive cancer within five years; inability to stop therapeutic doses of nonsteroidal anti-inflammatory drugs or anticoagulants; coagulopathy; contraindications to colonoscopy; chemotherapy within three months; high-risk conditions for colorectal cancer, such as familial adenomatous polyposis, the Lynch syndrome, or other cancer syndromes; previous colorectal cancer or adenoma; inflammatory bowel disease; or more than 2 first-degree relatives with colorectal neoplasia.” Of the 4482 individuals, 3764 completed all stool tests and colonoscopy. Median age was 65 years (age range: 50-80 years). Men comprised 48 percent of the study population. Of evaluable patients, 93.6 percent were white. SDT-1 (PreGen-Plus™ lineage) was performed in 2497 individuals. SDT-2 (Cologuard™ lineage) was performed in 217 individuals (“the manufacturer altered the SDT-1 assay, which prompted an unplanned interim analysis after 2497 patients. On the basis of these interim results, we stopped SDT-1 testing and began doing the SDT-2 test”).
The authors reported: “Sensitivity for screen-relevant neoplasms was 20% by SDT-1, 11% by Hemoccult (P = 0.020), 21% by HemoccultSensa (P = 0.80); sensitivity for cancer plus high-grade dysplasia did not differ among tests. Specificity was 96% by SDT-1, compared with 98% by Hemoccult (P<0.001) and 97% by HemoccultSensa (P = 0.20). Stool DNA test 2 detected 46% of screen-relevant neoplasms, compared with 16% by Hemoccult (P < 0.001) and 24% by HemoccultSensa (P < 0.001). Stool DNA test 2 detected 46% of adenomas 1 cm or larger, compared with 10% by Hemoccult (P < 0.001) and 17% by HemoccultSensa (P < 0.001). Among colonoscopically normal patients, the positivity rate was 16% with SDT-2, compared with 4% with Hemoccult (P = 0.010) and 5% with HemoccultSensa (P = 0.030).” They concluded: “Stool DNA test 1 provides no improvement over HemoccultSensa for detection of screen-relevant neoplasms. Stool DNA test 2 detects significantly more neoplasms than does Hemoccult or HemoccultSensa, but with more positive results in colonoscopically normal patients. Higher sensitivity of SDT-2 was particularly apparent for adenomas.”
Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, Butz ML, Thibodeau SN, Rabeneck L, Paszat LF, Kinzler KW, Vogelstein B, Bjerregaard NC, Laurberg S, Sørensen HT, Berger BM, Lidgard GP. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012 Feb;142(2):248-56; quiz e25-6. doi: 10.1053/j.gastro.2011.10.031. Epub 2011 Nov 4.
Ahlquist and colleagues reported the results of a case-control study to assess “colorectal neoplasm detection by a next-generation sDNA test and effects of covariates on test performance.” The aims of the study were to “(1) refine marker panel and individual marker cut-offs for optimal sDNA test discrimination, (2) assess clinical performance of the sDNA test in training and test sets, and (3) identify covariates that affect neoplasm detection rates.” Participants were selected from screening and diagnostic/surveillance settings. Patients with cancer or inflammatory bowel disease were excluded. Case stools were obtained from patients with colorectal cancer or at least one colorectal adenoma ≥ 1 centimeter (n = 252). Matched control stools were obtained from patients without neoplasia on colonoscopy (n = 293). The stool DNA test “detects 4 methylated genes, a mutant form of KRAS, and the -actin gene (as a reference value) using quantitative, allele-specific, real-time target and signal amplification; it also quantifies hemoglobin.” Logistic regression was used to model a training set (n = 456) and applied to a test set (n = 222). Median age was 61 years (age range: 39-92 years) in the training set and 60 years (age range: 40-88 years) in test set. Men comprised 50 percent of the training set and 51 percent of the test set. In the training set, 82 percent were white, while 80 percent were white in the test set.
The authors reported: “The sDNA test identified 85% of patients with CRC and 54% of patients with adenomas ≥ 1 cm with 90% specificity. The test had a high rate of detection for all nonmetastatic stages of CRC (aggregate 87% detection rate for CRC stages I-III). Detection rates increased with adenoma size: 54% ≥ 1 cm, 63% > 1 cm, 77% > 2 cm, 86% > 3 cm, and 92% > 4 cm (P < .0001).” They concluded: “Early-stage CRC and large adenomas can be detected throughout the colorectum and with high levels of accuracy by the sDNA test. Neoplasm size, but not anatomical site, affected detection rates. Further studies are needed to validate the findings in a larger population and optimize the sDNA test.”
Heigh RI, Yab TC, Taylor WR, Hussain FT, Smyrk TC4, Mahoney DW, Domanico MJ, Berger BM, Lidgard GP, Ahlquist DA. Detection of Colorectal Serrated Polyps by Stool DNA Testing: Comparison with Fecal Immunochemical Testing for Occult Blood (FIT). PLoS One. 2014 Jan 20;9(1):e85659. doi: 10.1371/journal.pone.0085659. eCollection 2014.
Heigh and colleagues reported the results of a nested case-control study in a larger cross sectional study “to assess the noninvasive detection of SSP [sessile serrated polyps] by stool DNA testing in asymptomatic persons undergoing screening or surveillance colonoscopic examination of the colon.” Specific aims were: “to (1) evaluate the performance of a pre-commercial multi-target stool DNA test (multi-target sDNA) for detection of SSP ≥ 1 cm, (2) determine which multi-target sDNA markers contribute most to SSP detection based on stool and tissue analyses, and (3) compare SSP detection rates by assay of exfoliated markers using stool DNA testing with those by assay of occult bleeding using a quantitative fecal immunochemical test (FIT).” Participants were asymptomatic adults scheduled for a screening or surveillance colonoscopy at two facilities in 2011 – 2012. Patients were excluded if they had “(1) a prior colorectal resection, (2) inflammatory bowel disease, Lynch syndrome, familial adenomatous polyposis, or other high risk conditions for CRC, (3) colonoscopy that was incomplete or associated with a poor preparation, or (4) a prior screening examination was done within 5 years.” Of the 456 participants, 29 had SSP ≥ 1 cm and served as cases while 232 had no detectable polyps and served as controls. Median ages were 62 years (age range: 57-77 years) in SSP cases and 61 years (range: 52-70) in controls. Men comprised 41 percent of SSP cases and 48 percent of controls.
The authors reported: “Among multi-target sDNA markers, mBMP3 proved highly discriminant for detection of SSP ≥ 1 cm (AUC = 0.87, p < 0.00001); other DNA markers provided no incremental sensitivity. Hemoglobin alone showed no discrimination (AUC = 0.50, p = NS). At matched specificities, detection of SSP ≥ 1 cm by stool mBMP3 was significantly greater than by FIT-50 (66% vs 10%, p = 0.0003) or FIT-100 (63% vs 0%, p < 0.0001).” They concluded that “[i]n a screening and surveillance setting, SSP ≥ 1 cm can be detected noninvasively by stool assay of exfoliated DNA markers, especially mBMP3. FIT appears to have no value in SSP
detection.”
Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N Engl J Med. 2014 Mar 19. [Epub ahead of print]
Imperiale and colleagues reported the results of a cross sectional study of 9989 individuals “to determine the performance characteristics of the DNA test in the detection of colorectal cancer.” Secondary aims were “to determine the performance of the DNA test in the detection of advanced precancerous lesions and to compare it with a commercially available fecal immunochemical test (FIT) for human hemoglobin in the detection of both colorectal cancer and advanced precancerous lesions.” Average risk individuals aged 50 to 84 years who were at average risk for colon cancer and were scheduled for screening colonoscopy were enrolled from June 2011 through November 2012 at 90 centers in the United States and Canada. Individuals “who had a personal history of colorectal neoplasia, digestive cancer, or inflammatory bowel disease; had undergone colonoscopy within the previous 9 years or a barium enema, computed tomographic colonography, or sigmoidoscopy within the previous 5 years; had positive results on fecal blood testing within the previous 6 months; had undergone colorectal resection for any reason other than sigmoid diverticula; had overt rectal bleeding within the previous 30 days; had a personal or family history of colorectal cancer; had participated in any interventional clinical study within the previous 30 days; or were unable or unwilling to provide written informed consent” were excluded. Primary outcome was detection of colorectal cancer. Secondary outcome was detection of “advanced precancerous lesions, including advanced adenomas (high-grade dysplasia or with ≥ 25% villous histologic features or measuring ≥ 1 cm in the greatest dimension) and sessile serrated polyps measuring 1 cm or more in diameter.”
Of the 12,776 participants, 9989 (78.2 percent) had results that could be fully evaluated. Mean age was 64 years. Men comprised 46 percent. Of the evaluable participants, 84 percent were Caucasian and 10.7 percent were African-American. The Cologuard™ test by Exact Sciences was used. It evaluates for six DNA markers and fecal hemoglobin and is reported as positive or negative (the test procedure and algorithm are seen in figures below). All participants underwent colonoscopy.
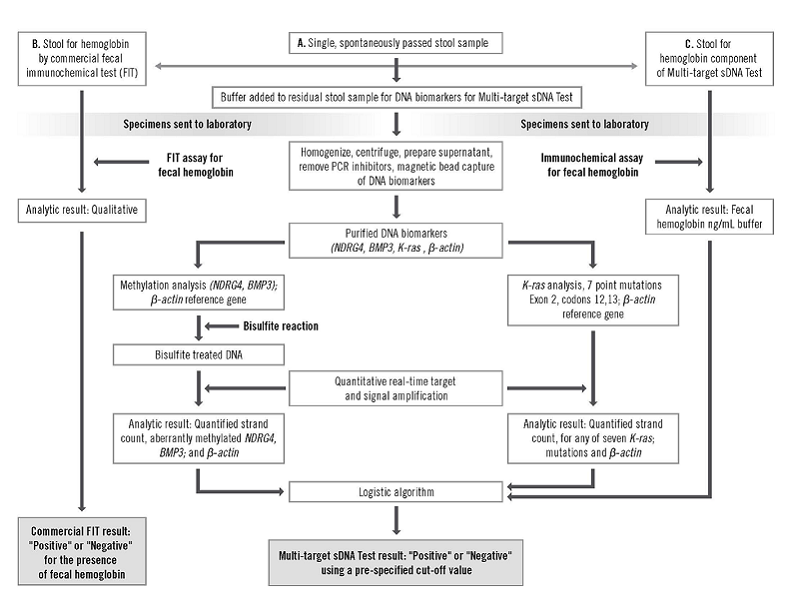
Figure S1. Study Specimen Flow and Approach to Extraction and Analysis of Stool DNA and Hemoglobin. Page 5. Supplement to: Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal cancer screening. N Engl J Med. DOI: 10.1056/NEJMoa1311194. Accessed 04/04/2014 at: http://www.nejm.org/doi/suppl/10.1056/NEJMoa1311194/suppl_file/nejmoa1311194_appendix.pdf
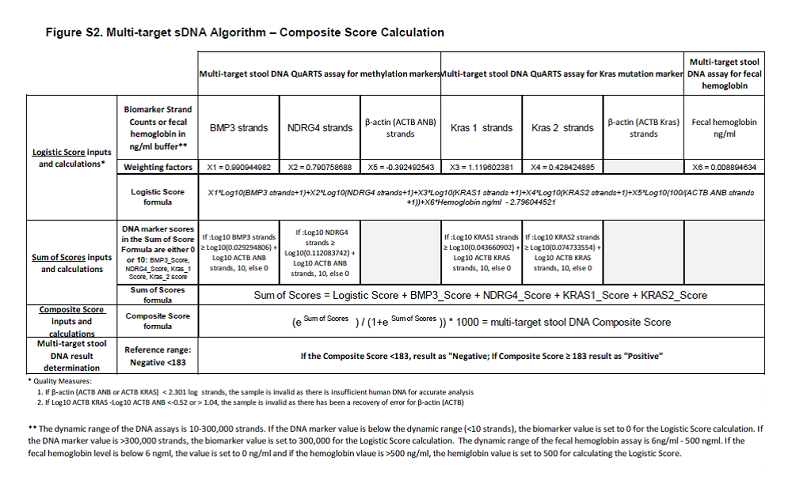
Figure S2. Multi-target sDNA Algorithm – Composite Score Calculation. Supplement to: Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal cancer screening. N Engl J Med. DOI: 10.1056/NEJMoa1311194. Accessed 04/04/2014 at: http://www.nejm.org/doi/suppl/10.1056/NEJMoa1311194/suppl_file/nejmoa1311194_appendix.pdf
The investigators found “65 (0.7%) had colorectal cancer and 757 (7.6%) had advanced precancerous lesions (advanced adenomas or sessile serrated polyps measuring ≥ 1 cm in the greatest dimension) on colonoscopy” and reported: “The sensitivity for detecting colorectal cancer was 92.3% with DNA testing and 73.8% with FIT (P = 0.002). The sensitivity for detecting advanced precancerous lesions was 42.4% with DNA testing and 23.8% with FIT (P < 0.001). The rate of detection of polyps with high-grade dysplasia was 69.2% with DNA testing and 46.2% with FIT (P = 0.004); the rates of detection of serrated sessile polyps measuring 1 cm or more were 42.4% and 5.1%, respectively (P < 0.001). Specificities with DNA testing and FIT were 86.6% and 94.9%, respectively, among participants with nonadvanced or negative findings (P < 0.001) and 89.8% and 96.4%, respectively, among those with negative results on colonoscopy (P < 0.001). The numbers of persons who would need to be screened to detect one cancer were 154 with colonoscopy, 166 with DNA testing, and 208 with FIT.” They concluded that “[i]n asymptomatic persons at average risk for colorectal cancer, multitarget stool DNA testing detected significantly more cancers than did FIT but had more false positive results.”
Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME; Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004 Dec 23;351(26):2704-14.
Imperiale and colleagues reported the results of a cross sectional study of 2507 individuals to compare “an approach that identifies abnormal DNA in stool samples with the Hemoccult II fecal occult-blood test in average-risk, asymptomatic persons 50 years of age or older.” There were no other inclusion criteria listed. Exclusion criteria included prior gastrointestinal bleeding, colorectal cancer, polyps and colonoscopy within 10 years. The fecal DNA test by Exact Sciences (PreGen-Plus™) was used. Colonoscopy was the reference standard test for cancers and polyps.
The fecal DNA test consisted of a panel “of 21 mutations: 3 in the KRAS gene, 10 in the APC gene, and 8 in the p53 gene; the microsatellite instability marker BAT-26; and a marker of long DNA thought to reflect disordered apoptosis of cancer cells sloughed into the colonic lumen.”
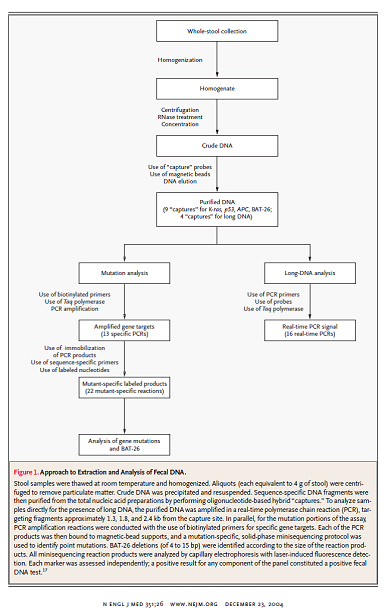
Figure I. Page 2707. N Engl J Med. 2004 Dec 23;351(26);2704-14.
Of the 5486 individuals enrolled, 4404 completed stool tests and colonoscopy. Of these, 2507 were included in the analysis (23 patients with advanced adenomas were excluded, as well as 1874 randomly selected patients with minor polyps or no polyps). For the 2507 patients analyzed, mean age was 69.5 years. Men comprised 44.5 percent of the analyzed group. Of the evaluable participants 87.3 percent were white and 8.4 percent were black.
The authors reported: “The fecal DNA panel detected 16 of 31 invasive cancers, whereas Hemoccult II identified 4 of 31 (51.6 percent vs. 12.9 percent, P = 0.003). The DNA panel detected 29 of 71 invasive cancers plus adenomas with high-grade dysplasia, whereas Hemoccult II identified 10 of 71 (40.8 percent vs. 14.1 percent, P < 0.001). Among 418 subjects with advanced neoplasia (defined as a tubular adenoma at least 1 cm in diameter, a polyp with a villous histological appearance, a polyp with high-grade dysplasia, or cancer), the DNA panel was positive in 76 (18.2 percent), whereas Hemoccult II was positive in 45 (10.8 percent). Specificity in subjects with negative findings on colonoscopy was 94.4 percent for the fecal DNA panel and 95.2 percent for Hemoccult II.” They concluded: “Although the majority of neoplastic lesions identified by colonoscopy were not detected by either noninvasive test, the multitarget analysis of fecal DNA detected a greater proportion of important colorectal neoplasia than did Hemoccult II without compromising specificity.”
Lidgard GP, Domanico MJ, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, Allawi H, Yab TC, Taylor WR, Simonson JA, Devens M, Heigh RI, Ahlquist DA, Berger BM. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1313-8. doi: 10.1016/j.cgh.2013.04.023. Epub 2013 Apr 29.
Lidgard and colleagues reported the results of a case-control study “to evaluate the clinical performance of the automated system for the detection of the critical lesions targeted in CRC screening: curable-stage CRC, advanced adenoma (AA), and sessile serrated adenoma > 1 cm (SSA).” Participants were scheduled for screening or surveillance colonoscopy. There were 207 cases and 796 controls. Median age was 65 years in cases (age range: 38-87 years) and controls (age range: 50-84). Men comprised 58 percent of cases and 42 percent of controls. “Cases included CRC (n = 93), advanced adenoma (AA) (n = 84), or sessile serrated adenoma ≥ 1 cm (SSA) (n = 30); controls included nonadvanced polyps (n = 155) or no colonic lesions (n = 641).”
The authors reported: “At 90% specificity, sDNA analysis identified individuals with CRC with 98% sensitivity. Its sensitivity for stage I cancer was 95%, for stage II cancer it was 100%, for stage III cancer it was 96%, for stage IV cancer it was 100%, and for stages I–III cancers it was 97% (nonsignificant P value). Its sensitivity for advanced precancers (AA and SSA) ≥ 1 cm was 57%, for > 2 cm it was 73%, and for > 3 cm it was 83%. The assay detected AA with high-grade dysplasia with 83% sensitivity.” They concluded: “We developed an automated, multi-target sDNA assay that detects CRC and premalignant lesions with levels of accuracy previously demonstrated with a manual process. This automated high-throughput system could be a widely accessible noninvasive approach to general CRC screening.”
Skally M, Hanly P, Sharp L. Cost effectiveness of fecal DNA screening for colorectal cancer: a systematic review and quality appraisal of the literature. Appl Health Econ Health Policy. 2013 Jun;11(3):181-92. doi: 10.1007/s40258-013-0010-8.
Skally and colleagues reported the results of a systematic review and quality assessment of fecal DNA (fDNA) cost-effectiveness studies “to systematically review the evidence base on cost-effectiveness of fDNA as a colorectal cancer screening tool—in comparison with no screening and with other available screening modalities— and to assess a range of model input parameters in order to identify key variables that impinged on cost-effectiveness.” Eligible studies included those that undertook an economic evaluation of fDNA in individuals of average risk, using either a cost-effectiveness or cost-utility analysis, with fDNA used as a primary screening comparator to other relevant screening modalities and/or no screening based on incremental cost-effectiveness ratios (ICERs).” “Additional inclusion criteria related to the presentation of data pertaining to model variables including time horizon, costs, fDNA performance characteristics, screening uptake, and comparators.” Of the 369 eligible papers identified from January 2002 to September 2011, seven met inclusion criteria and were reviewed.
The authors reported: “Compared with other screening modalities, fDNA was not considered cost-effective in any of the base-case analyses: in five studies it was dominated by all alternatives considered. Sensitivity analyses identified cost, compliance, and test parameters as key influential parameters.” They concluded: “On the basis of the available (albeit limited) evidence, while fDNA is cost-effective when compared with no screening, it is currently dominated by most of the other available screening options. Cost and test performance appear to be the main influences on cost-effectiveness.”
Zou H, Allawi H, Cao X, Domanico M, Harrington J, Taylor WR, Yab T, Ahlquist DA, Lidgard G. Quantification of methylated markers with a multiplex methylation-specific technology. Clin Chem. 2012 Feb;58(2):375-83. doi: 10.1373/clinchem.2011.171264. Epub 2011 Dec 22.
Zou and colleagues reported the results of a comparative study to “(a) test the analytical sensitivity of QuARTS [quantitative allele-specific real-time target and signal amplification] for detecting low abundance methylated gene copies against excessive background amounts of unmethylated copies and (b) assess the QuARTS technology by multiplex detection of tumor-specific methylated genes BMP3 (bone morphogenetic protein 3)4, NDRG4 (N-myc downstream-regulated 4), VIM (vimentin), TFPI2 (tissue factor pathway inhibitor 2), and a reference gene ACTB (β-actin) in well-characterized tissues from patients with colorectal cancers or advanced adenomas (≥ 1 cm) and from individuals with normal colonoscopies.” The technology was tested “on 91 DNA samples extracted from colorectal tissues, including 37 cancers, 25 adenomas, and 29 healthy epithelia” obtained during colonoscopy.
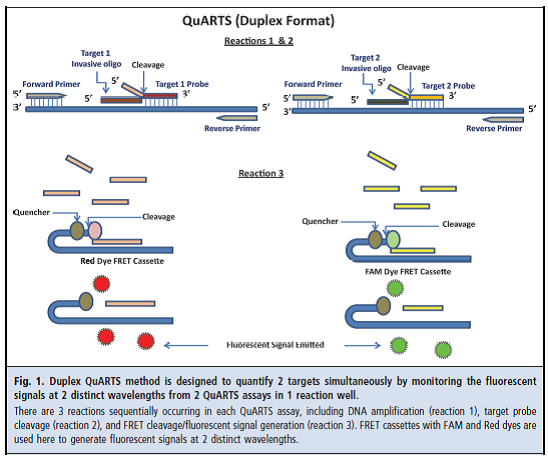
Figure 1. Page 376. Zou et al.. Quantification of methylated markers with a multiplex methylation-specific technology. Clin Chem. 2012 Feb;58(2):375-83.
The authors reported: “The QuARTS method linearly detected methylated or unmethylated VIM gene down to 10 copies. No cross-reactivity was observed when methylated assays were used to amplify 105 copies of unmethylated gene and vice versa. The multiplex assay detected methylated genes spiked in unmethylated genes at a 0.01% ratio and vice versa. At a diagnostic specificity cutoff of 95%, methylated BMP3, NDRG4, VIM, and TFPI2 detected 84%, 92%, 86%, and 92% of colorectal cancers and 68%, 76%, 76%, and 88% of adenomas, respectively.”
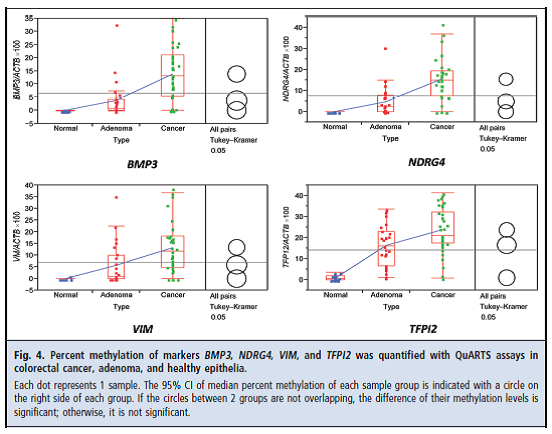
Figure 4. Page 380. Zou et al. Quantification of methylated markers with a multiplex methylation-specific technology. Clin Chem. 2012 Feb;58(2):375-83.
They concluded: “The QuARTS technology provides a promising approach for quantifying methylated markers. The markers assayed highly discriminated colorectal neoplasia from healthy epithelia.”
3. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
The MEDCAC was not convened on this topic.
4. Evidence-Based Guidelines
U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008 Nov 4;149(9):627-37. Epub 2008 Oct 6.
The following recommendations related to colorectal cancer screening were issued by the USPSTF.
- “The USPSTF recommends screening for colorectal cancer using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults, beginning at age 50 years and continuing until age 75 years. The risks and benefits of these screening methods vary. (Grade: A recommendation)
- The USPSTF recommends against routine screening for colorectal cancer in adults 76 to 85 years of age. There may be considerations that support colorectal cancer screening in an individual patient. (Grade: C recommendation)
- The USPSTF recommends against screening for colorectal cancer in adults older than age 85 years. (Grade: D recommendation)
- The USPSTF concludes that the evidence is insufficient to assess the benefits and harms of computed tomographic colonography and fecal DNA testing as screening modalities for colorectal cancer. (Grade: I statement).” We note that this recommendation was specific to a predecessor test. The Cologuard™ test was not evaluated.
Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008 Nov 4;149(9):638-58. Epub 2008 Oct 6.
Whitlock and colleagues reported the results of a systematic review to inform the USPSTF recommendations. Specifically for fecal DNA testing, the authors reported: “Eligible fecal DNA screening studies were limited to a fair-quality large cohort study that used a multitarget fecal DNA panel test (the precommercial version of PreGen Plus, version 1 [Exact Sciences, Marlborough, Massachusetts], which tests for 21 DNA mutations in the K-ras, APC, and p53 genes, along with markers for microsatellite instability and long DNA) in average-risk patients undergoing colonoscopy (47) and a smaller cohort study that tested a single mutation of the K-ras gene (48). We will not further discuss the test for the single K-ras gene mutation because it showed zero sensitivity: It was positive in none of the 31 participants with advanced colorectal neoplasia, including 7 patients with invasive colorectal cancer. Researchers compared a one-time application of Pre-Gen Plus (version 1.0) with 3-card nonrehydrated Hemoccult II in a study that enrolled 5486 average-risk asymptomatic patients who were all to undergo colonoscopy (47) (Table 1). Among the 4404 that adhered to all 3 tests, a subset (n = 2507; mean age, 69.5 years; 45% male; 87% white; 14% with a positive family history) was selected for fecal DNA testing on the basis of colonoscopic and histopathologic results. Test performance for fecal DNA was compared with that for Hemoccult II in the selected subgroup; among these patients, 8.2% had positive results on the fecal DNA panel and 5.8% had positive Hemoccult II results. One time fecal DNA testing was more sensitive for adenocarcinoma than was Hemoccult II (sensitivities of 51% [CI, 34.8% to 68.0%] and 12.9% [CI, 5.1% to 28.9%], respectively). Both fecal DNA testing and Hemoccult II had poor sensitivity for advanced carcinoma. Although specificity for minor polyps or no polyps did not differ between fecal DNA and Hemoccult II, power to detect a difference may have been limited because the full sample was not tested.”
This recommendation was specific to the PreGen-Plus™ test. The Cologuard™ test was not evaluated.
5. Professional Society Recommendations/Consensus Statements
Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, Johnson CD, Levin TR, Pickhardt PJ, Rex DK, Smith RA, Thorson A, Winawer SJ; American Cancer Society Colorectal Cancer Advisory Group; US Multi-Society Task Force; American College of Radiology Colon Cancer Committee. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008 May;134(5):1570-95. doi: 10.1053/j.gastro.2008.02.002. Epub 2008 Feb 8.
Excerpt from table 2 “Guidelines for Screening for the Early Detection of Colorectal Cancer and Adenomas for Average-risk Women and Men Aged 50 Years and Older”
Specifically for fecal DNA testing: “sDNA—Conclusions and Recommendations. In previous assessments of the performance of sDNA, both the ACS and the USMSTF concluded that data were insufficient to recommend screening with sDNA for average-risk individuals. Based on the accumulation of evidence since the last update of these guidelines, the panel concluded that there now are sufficient data to include sDNA as an acceptable option for CRC screening. As noted above, testing stool for molecular markers is an evolving technology. New iterations of these tests, either technological enhancements of existing tests or completely new test variants, should be carefully evaluated in order to determine that they meet the criteria of detecting a majority of cancers at the time of screening but also have acceptable performance in a screening cohort. While the manufacturer of the one test that is commercially available currently is recommending a 5-year interval for routine screening between examinations with normal results, the panel concluded that there were insufficient data upon which to endorse this interval. Such an interval was judged by the committee to be appropriate only for a test that has very high sensitivity for both cancer and adenomatous polyps—a standard that has not been documented for sDNA to date. At this time, further research is needed to determine the interval between negative sDNA exams. Based on current evidence, the appropriate interval is uncertain.”
This recommendation was based on a predecessor test. The Cologuard™ test was not evaluated.
Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009 Mar;104(3):739-50. doi: 10.1038/ajg.2009.104. Epub 2009 Feb 24.
![Excerpt Table 3 (partial for average risk individuals). Grade 2 B was defined as “[w]eak recommendation, moderate quality evidence. Benefits closely balanced with risks and burden. RCTs with important limitations (inconsistent results, methodological flaws, indirect, or imprecise) or exceptionally strong evidence from observational studies. Weak recommendation, best action may differ depending on circumstances or patients or societal values.”](http://www.cms.gov/medicare/coverage/determinationprocess/other-content-types/id277image013png.png)
Excerpt Table 3 (partial for average risk individuals). Grade 2 B was defined as “[w]eak recommendation, moderate quality evidence. Benefits closely balanced with risks and burden. RCTs with important limitations (inconsistent results, methodological flaws, indirect, or imprecise) or exceptionally strong evidence from observational studies. Weak recommendation, best action may differ depending on circumstances or patients or societal values.”
Specifically for fecal DNA testing: “Fecal DNA testing has been evaluated in three different versions. The first (Version 1.0) included tests for point mutations in k-ras, APC, P53, mutations in the BAT26 microsatellite instability marker, and the DNA integrity assay. The sensitivity for cancer was superior to traditional guaiac-based occult blood testing, but the absolute sensitivity was 52% and disappointing considering the high cost of the test (130). After completion of the trial, it was learned that the DNA integrity assay, which had appeared to be the most promising element in the assay in early studies (131), was non-informative because of the instability of DNA during shipment. Subsequently, Version 1.1 has been commercialized, which includes the same DNA test used in Version 1.0, but includes technical improvements of gel-based DNA capture and buffer stabilization of long or redundant DNA critical to the DNA integrity assay. No screening test using Version 1.1 has been reported, but a trial in established CRCs identified 70% sensitivity and specificity of ~ 95% , (specificity similar to Version 1.0) (132). Version 2.0 utilizes a simplified assay consisting of the DNA integrity assay and hypermethylation of the vimentin gene. No screening trial with Version 2.0 has been carried out, but a study in established CRCs shows sensitivity of 87% for cancer, but specificity fell to 82% (133). The latter specificity limits the frequency with which the test can be carried out reasonably. Given that the performance characteristics of the FIT are approximately equal to Versions 1.0, and 1.1, and superior to Version 2.0 with regard to specificity, and that FIT costs much less than fecal DNA testing, there is no rationale for primary use of fecal DNA testing as a CRC detection test. The value of combining FIT and fecal DNA testing is unknown. Additional disadvantages of fecal DNA testing include no established data on which to determine an optimal interval, and the lack of clinical recommendations on how to respond to patients who have positive DNA tests and negative colonoscopies. Although the recent guideline endorsing fecal DNA testing declined to recommend an interval for DNA testing, the ACG considers that testing at intervals < 3 years would be cost prohibitive.”
This recommendation was based on a predecessor test. The Cologuard™ test was not evaluated.
Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, Wender RC, Brawley OW. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014 Jan-Feb;64(1):30-51. doi: 10.3322/caac.21212. Epub 2014 Jan 9.
Smith and colleagues reported updated guidelines for cancer screening. Specifically for stool DNA testing:
b. “The stool DNA test approved for colorectal cancer screening in 2008 is no longer commercially available. New stool DNA tests are presently undergoing evaluation and may become available at some future time.”
Excerpt from “Table 2. ACS Recommendations for the Early Detection of Cancer in Average-Risk, Asymptomatic Individuals.”
This recommendation was based on a predecessor test. The Cologuard™ test was not evaluated.
VIII. CMS Analysis
National coverage determinations are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1862(l) of the Act). Among other things, in order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. CRC screening tests have a benefit category under §1832, §1861(s)(2)(R) and §1861(pp) of the Act. Specifically, CMS is using the authority under section 1861(pp)(1)(D) and 42 CFR 410.37(a)(1)(v) to determine whether the scope of the CRC screening benefit should be expanded to include coverage of the Cologuard™ sDNA test.
Question 1: Is the evidence sufficient to determine that the CologuardTM test is a suitable colorectal cancer screening test for prevention or early detection in Medicare beneficiaries?
Due to the natural history of colorectal neoplasia, the burden of CRC and large pre-cancerous adenomas is high in older adults. Of the currently covered, non-invasive fecal CRC screening tests, test performance and utilization are suboptimal. In this analysis, we will evaluate specific criteria of screening tests as described by Cochrane and Holland (1971) and noted in the introductory paragraphs of the evidence (See section VII of this proposed decision memorandum).
In 2008, we started a review of the PreGen-Plus™ test as a CRC screening test. However, a decision was deferred at the time since the test was yet to be commercially available in the United States market. Since then, there have been a number of advancements in technology and refinements in the test itself. Some of the initial evidence on PreGen-Plus™ is useful to include in consideration of the latest generation test, Cologuard™, since it illustrates the progression of sDNA testing as a colorectal cancer screening test and offers support to the plausibility and feasibility of this technology. PreGen-Plus™ consisted of a panel of 21 mutations of K-ras, APC and p53. During testing, PreGen-Plus™ was refined to a smaller number of specific mutations with DNA normalization and inclusion of a FIT component, subsequently becoming the currently available sDNA test.
Three observational studies on Cologuard™ provided evidence for this analysis. Two observational studies on predecessor tests were included. The strongest evidence for Cologuard™ was provided by the study by Imperiale (2014) which included 9989 evaluable participants (median age was 67 years) and was performed at 90 clinical sites in the United States and Canada. It was a well-designed, well-conducted cross-sectional study with comparison to colonoscopy, the current standard, and FIT. The Cologuard™ test had a significantly higher sensitivity for colorectal cancer and advanced precancerous lesions compared to FIT; while specificity was significantly lower (more false positive results; Imperiale, 2014)). A cross-sectional study by Ahlquist (2008) evaluated a predecessor test but also provided initial data on a refined version (n = 217) of the test. An additional case-control study by Ahlquist (2012) served as a supportive, early exploratory study.
Across comparative studies, the Cologuard™ test appeared to perform better than a predecessor test. A summary of test characteristics is presented in the table below.
| |
sensitivity (95% CI) |
specificity (95% CI) |
NNS |
| |
colorectal cancer |
advanced adenoma -. precancerous lesions |
negative colonoscopy |
|
|---|
| Imperiale, 2014 |
|
|
|
|
| Cologuard™ |
92.3 (83.0-97.5) |
42.4 (38.9-46.0) |
89.8 (88.9-90.7) |
166a |
| FIT |
73.8 (61.5-84.0) |
23.8 (20.8-27.0) |
96.4 (95.8-96.9) |
208 |
| Ahlquist, 2008 (note) |
|
< |
|
|
| Hemoccult |
11 (6-16) |
not reported |
98 (8-99) |
not reported |
| HemoccultSensa |
21 (15-27) |
not reported |
97 (96-97) |
not reported |
| sDNA1 |
20 (14-26) |
not reported |
96 (95-97) |
not reported |
| sDNA2 |
40 (32-49) |
not reported |
not reported |
not reported |
| Imperiale, 2004 |
|
|
|
|
| predecessor |
51.6 (34.8-68.0) |
15.1 (12.0-19.0) |
94.4 |
not reported |
| FOBT (Hemoccult II) |
12.9 (5.1-28.9) |
10.7 (8.0-14.1) |
95.2 |
not reported |
| Exact Sciences, 2014 |
|
|
|
|
| Cologuard™ ≥ 65 yrs |
92.6 |
44.6 |
83.8 |
126b |
Values from the Ahlquist study were combined for cancer, high grade dysplasia and adenomas ≥ 1 cm. (a) NNS = number needed to screen to detect one cancer (reference colonoscopy = 154); advanced precancerous lesions include 2014). (b) NNS = number needed to screen to detect one cancer (reference colonoscopy = 117; FIT = 158) (Barry Berger from Exact Sciences).
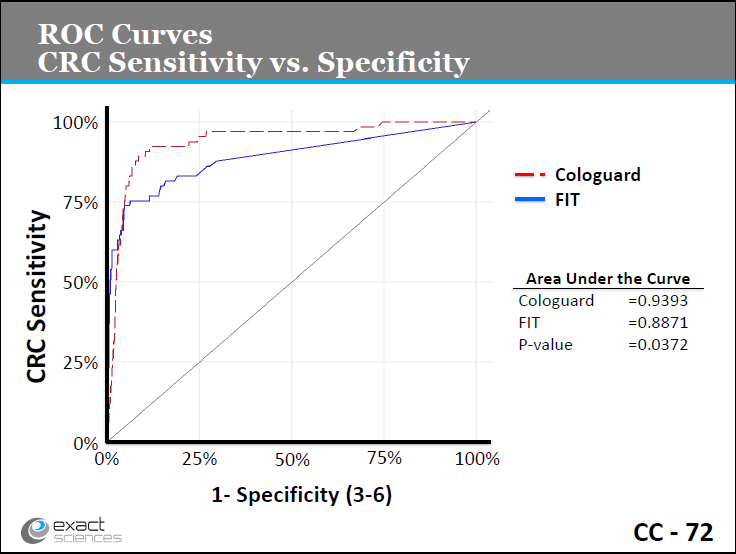
Slide CC-72. Exact Sciences presentation, March 27, 2014.
http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MolecularandClinicalGeneticsPanel/UCM391101.pdf
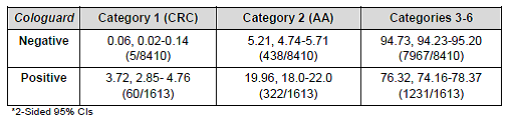
Table 18. Predictive values. Page 52. Exact Science FDA SSED. Category 2 (advanced adenomas); categories 3-6 (adenomas < 10 mm or negative)
In the Imperiale study (2014), Cologuard™ detected 12 more cancers and 141 more advanced lesions than FIT while having 293 more false positive results (293 more individuals referred for further diagnostic evaluation with colonoscopy, and that were found negative on colonoscopy). The study also provided evidence of the advancing technology and methodology of stool DNA testing using Cologuard™ from its predecessor test, PreGen-Plus™, which itself was an improvement over FOBT (Imperiale, 2004). Biological plausibility of the individual markers in Cologuard™ has been shown in prior investigational studies. The Imperiale study (2014) provided evidence that a production multitarget sDNA test can function as a CRC screening test.
The increased detection of advanced precancerous lesions has the potential to translate into a substantial public health benefit in primary prevention of CRC, further reducing CRC incidence and mortality. Like other fecal based tests, Cologuard™ is simple and acceptable (Schroy, 2005). Accuracy, precision and reproducibility have been shown in published studies and in data presented to the FDA (Summary of Safety and Effectiveness Data (SSED)).
The main limitation of the published studies lies in the cross-sectional design. While the cross-sectional design is valid and commonly used for comparing a new screening test to a proven gold standard, direct evidence on net health outcomes may be limited if there is no additional follow-up period. Fortunately for CRC screening, this is substantially addressed by the ample evidence from prior screening studies using FOBT and colonoscopy that demonstrated improvements in health outcomes. Early detection of CRC using FOBT has been shown to improve colorectal cancer mortality (Mandel, 1993; Shaukat, 2013). Early detection and removal of large precancerous adenomas has been shown to prevent (reduce incidence of) CRC (Mandel, 2000; Nishihara, 2013). As another fecal based test, screening with the Cologuard™ test will likely improve health outcomes by early detection of precancerous adenomas and CRC at earlier stages. Diagnostic and therapeutic colonoscopy is a required step in the pathway to improved outcomes with all non-invasive fecal screening tests.
The optimal interval of screening is also difficult to determine from cross-sectional studies. The manufacturer has committed to conduct a follow-up post approval study, as required by the FDA, to generate additional evidence to address longer term outcomes and frequency. Using a test that may be performed at less frequent intervals than annual FOBT or FIT, compliance with a sustained screening program may also be increased (Mandel, 2000).
Like other non-invasive, fecal based CRC screening tests (guaiac FOBT, FIT), the harms from the sDNA test itself are likely to be small. The associated harms lie in the downstream risks from diagnostic and therapeutic colonoscopy after a positive sDNA test. The harms from colonoscopy have been well documented and are generally considered low (in a retrospective analysis of 43,456 screening and diagnostic colonoscopies, Rutter (2012) reported a 30-day serious adverse event rate of 0.49 percent. Serious adverse events included perforation, hemorrhage, and diverticulitis). A related harm (anxiety/concern) may arise in individuals with a positive fecal test but a negative colonoscopy, a scenario that also presents a follow-up and surveillance dilemma for clinicians as well. In past considerations of fecal based tests, the benefits have been judged to outweigh the risks/harms (Levin, 2008; USPSTF, 2008).
Overall, the Cologuard™ test is a suitable screening test for CRC, as demonstrated primarily in a large well conducted study (Imperiale, 2014) and has significantly higher sensitivity compared to the other currently covered fecal screening tests. As a suitable screening test likely to lead to improvements in health outcomes, the evidence is sufficient to conclude that CRC screening in Medicare beneficiaries using the Cologuard™ test is appropriate for prevention or early detection. This is consistent with the United States Multi-Society Task Force (2008), American College of Radiology and American Cancer Society (2008) who have already added sDNA testing to their list of recommended CRC screening tests based on a predecessor test. The American College of Gastroenterology (2009) and USPSTF (2008) have an insufficient grading, but are likely to reconsider based on the new data from Imperiale (2014).
Question 2: Is the evidence sufficient to determine that colorectal cancer screening using the Cologuard™ test is appropriate for Medicare beneficiaries?
Previous research has shown that CRC screening has been shown to reduce CRC incidence and mortality and is recommended for the Medicare aged population. The USPSTF recommends CRC screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults aged 50-75 years (Grade: A recommendation; USPSTF, 2008). At that time, the task force stated that the evidence is insufficient to assess the benefits and harms of fecal DNA testing, noting that “[f]ecal DNA tests are evolving, and no test is widely used.” With FDA approval of Cologuard™ and increased availability of the test, the USPSTF will likely re-evaluate CRC screening using this modality. The American Cancer Society, U.S. Multisociety Task Force on Colorectal Cancer (including American College of Gastroenterology, American Gastroenterological Association Institute, and American Society for Gastrointestinal Endoscopy), and the American College of Radiology recommend CRC screening for asymptomatic, average risk adults age 50 years and older using a menu of options including annual FOBT screening and stool DNA test, each with high sensitivity for cancer, interval uncertain (Levin, 2008). The new study results may allow refinement and strengthening of the recommendation grades.
While the age inclusion criterion in the study by Imperiale (2014) was 50-84 years, the study enrollment was specifically enhanced to focus on older adults and to increase generalizability of results to the Medicare population (of which 85 percent are 65 years or older). With this design, 6308 individuals aged 65 years and older (63 percent) were included in the analyzable dataset. Based on the over-sampling of older adults, the findings of the Imperiale study are directly applicable. The study was also conducted at 90 centers across the United States and Canada, reducing the concern of geographic variability while demonstrating the feasibility of widespread implementation. Based on the potential impact of the availability of a newly FDA approved sDNA test with enhanced test performance, professional society recommendations, clinical guidelines, and newly published studies, we believe that the evidence is sufficient to determine that colorectal cancer screening using the Cologuard™ test is appropriate for the Medicare population.
Frequency of Screening
The frequency of CRC screening with the Cologuard™ test has not been definitively established. Since cross-sectional studies usually provide evidence at one point in time (one screening in this case), these studies do not provide direct evidence on how often any particular test should be performed. The manufacturer of the current FDA approved sDNA test has suggested CRC screening once every three years with Cologuard™. The post approval study required by the FDA is designed to evaluate the validity of screening every three years to ensure that clinically important findings are not missed. With the natural history of most colorectal neoplasia and improved test performance, screening every three years appears to be a reasonable initial frequency to detect clinically meaningful changes. CMS will re-evaluate the screening interval after the completion of the post approval study and modify coverage if appropriate.
In the interim period, CMS encourages eligible Medicare beneficiaries to continue to participate in recommended CRC screening, as appropriate. For example, Medicare covers annual FIT testing, which has been shown to reduce CRC incidence and mortality.
CRC Screening Approaches
Professional organizations have recommended a menu of options in terms of CRC screening tests to increase screening participation. Since a number of factors may be involved in the choice of screening tests, having several available tests may assist providers and beneficiaries to personalize individual screening approaches. Having providers assist beneficiaries in choosing among the available screening options may also help reduce over-utilization and redundant screening. The sDNA test every three years (or other subsequent interval once the FDA required post approval study is completed) provides an intermediate option between FIT every year and colonoscopy every 10 years. This interval has not been previously available with a non-invasive fecal screening test, potentially increasing CRC screening participation overall. Eligible beneficiaries may continue to participate in CRC screening strategies that include annual FIT and colonoscopy every 10 years as recommended by their physicians.
Age Range
The USPSTF recommends CRC screening using fecal occult blood testing, sigmoidoscopy, or colonoscopy in adults aged 50-75 years (Grade: A recommendation); recommends against routine CRC screening in adults aged 76-85 years (there may be considerations that support CRC screening in an individual patient) (Grade: C recommendation); and recommends against CRC screening in adults older than age 85 years (Grade: D recommendation). The study by Imperiale (2014) enrolled adults aged 50 to 84 years. We believe, based on the evidence reviewed, including professional clinical guidelines, that Cologuard™ testing in adults at average risk of developing colorectal cancer, aged 50 to 85 years is appropriate.
Expenditures
As permitted by §1861(pp)(1)(D) of the Act, we may consider the appropriate frequency and payment level in determining whether to expand coverage for new CRC tests. CMS has commissioned such analyses in all past determinations (FIT, stool DNA, and computed tomography (CT) colonography) and will reanalyze using the test parameters of Cologuard™.
Disparities
Colorectal cancer disproportionately affects adults 50 years of age and older. Enrollment of older adults in the study by Imperiale (2014) was specifically enhanced to increase generalizability to the Medicare population, and enrolled representative samples of women and minorities based on United States census data. Given the burden of CRC, CMS encourages all eligible Medicare beneficiaries to participate in CRC screening.
Summary
Mortality rates for CRC have declined over the past decade, but CRC was estimated to account for over 50,000 deaths in 2013, with a median age at death of 74 years (NIH/SEER 2013). Primary prevention, early detection and early treatment have contributed to the observed reduction in mortality. Medicare currently covers several CRC screening tests yet utilization rates are suboptimal. The Cologuard™ test is a technologically advanced, non-invasive CRC screening test with test performance that has been shown to be significantly better in detecting advanced adenomas and cancers than current fecal immunochemical tests (Imperiale, 2014). Based on a systematic review of the evidence, we propose that colorectal cancer screening in Medicare beneficiaries using the Cologuard™ test is appropriate for the prevention or early detection of illness or disability.
IX. Conclusion
The Centers for Medicare & Medicaid Services (CMS) proposes that the evidence is sufficient to cover Cologuard™ – a multitarget stool DNA test – as a colorectal cancer screening test for asymptomatic average risk beneficiaries aged 50 to 85 years.
Therefore, CMS proposes to cover the Cologuard™ test once every three years for beneficiaries who meet all of the following criteria:
- Age 50 to 85 years,
- Asymptomatic (no signs or symptoms of colorectal disease including but not limited to lower gastrointestinal pain, blood in stool, positive guaiac fecal occult blood test or fecal immunochemical test), and
- At average risk of developing colorectal cancer (no personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease, including Crohn’s Disease and ulcerative colitis; no family history of colorectal cancers or an adenomatous polyp, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer).
CMS is seeking comments on our proposed decision. We will respond to public comments in a final decision memorandum, as required by §1862(l)(3) of the Act.
Appendix A
General Methodological Principles of Study Design
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is appropriate for coverage under the Medicare program. The critical appraisal of the evidence enables us to determine whether: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients. An improved health outcome is one of several considerations in determining whether an item or service is appropriate.
CMS divides the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the relevance of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s risks and benefits.
The issues presented here represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has unique methodological aspects.
1. Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematic assessment of factors related to outcomes.
- Larger sample sizes in studies to help ensure adequate numbers of patients are enrolled to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to which group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is the extent to which differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias),
- Co-interventions or provision of care apart from the intervention under evaluation (confounding),
- Differential assessment of outcome (detection bias), and
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The following is a representative list of study designs (some of which have alternative names) ranked from most to least methodologically rigorous in their potential ability
to minimize systematic bias:
- Randomized controlled trials,
- Non-randomized controlled trials,
- Prospective cohort studies,
- Retrospective case control studies,
- Cross-sectional studies,
- Surveillance studies (e.g., using registries or surveys),
- Consecutive case series, and
- Single case reports.
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in which confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly a study’s selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess the evidence.
2. Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens, and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to which the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease, and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing, and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage decisions for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation), and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations because one of the goals of our determination process is to assess health outcomes. We are interested in the results of changed patient management not just altered management. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
3. Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not appropriate if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is appropriate. For most determinations, CMS evaluates whether reported benefits translate into improved health outcomes. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
CMS does from time to time include requirements for facility and/or physician standards, or certain certification requirements in our NCDs; however, we exercise this option after considered counsel and are cognizant of the responsibility such requirements establish. Some of the considerations that may inform our decision to include facility and/or physician standards, or certification requirements are: intended patients who are medically fragile undergoing high risk procedures; procedures that are new or not generally disseminated in the medical community at large; technically complex procedures; procedures experiencing a rapid growth in the medical community before the opportunity for the establishment of generally accepted standards; procedures that impose what we believe to be a significantly higher risk for our Medicare beneficiaries. While this is not intended to be an all-inclusive list of what may inform CMS’s decision to include facility and/or physician standards, or certification requirements, it is provided to give some insight into our decision making process. Ultimately, it is the convincing nature of the circumstances and/or the evidence surrounding the item or service under review that guides CMS to conclude that such standards and/or certification requirements will benefit our Medicare beneficiaries.
Appendix B
Articles Submitted by the Requestor
Ahlquist DA, Sargent DJ, Loprinzi CL, Levin TR, Rex DK, Ahnen DJ, Knigge K, Lance MP, Burgart LJ, Hamilton SR, Allison JE, Lawson MJ, Devens ME, Harrington JJ, Hillman SL. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008 Oct 7; 149(7):441-50, W81.
Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000 Nov;119(5):1219-27.
Ahlquist DA, Taylor WR, Mahoney DW, Zou H, Domanico M, Thibodeau SN, Boardman LA, Berger BM, Lidgard GP. The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol. 2012 Mar;10(3):272-7.e1. Epub 2011 Oct 20.
Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, Butz ML, Thibodeau SN, Rabeneck L, Paszat LF, Kinzler KW, Vogelstein B, Bjerregaard NC, Laurberg S, Sørensen HT, Berger BM, Lidgard GP. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012 Feb;142(2):248-56; quiz e25-6. Epub 2011 Nov 4.
Brand RE, Ross ME, Shuber AP. Reproducibility of a multitarget stool-based DNA assay for colorectal cancer detection. Am J Gastroenterol. 2004 Jul;99(7):1338-41.
Calistri D, Rengucci C, Bocchini R, et al. Fecal multiple molecular tests to detect colorectal cancer in stool. Clin Gastroenterol Hepatol. 2003 Sep;1(5):377-83.
Diehl F, Schmidt K, Durkee KH, Moore KJ, Goodman SN, Shuber AP, Kinzler KW, Vogelstein B. Analysis of mutations in DNA isolated from plasma and stool for colorectal cancer patients. Gastroenterology. 2008 Aug;135(2):489-98. Epub 2008 May 15.
Haug U, Hillebrand T, Bendzko P, et al. Mutant-enriched PCR and allele-specific hybridization reaction to detect K-ras mutations in stool DNA: high prevalence in a large sample of older adults. Clin Chem. 2007 Apr;53(4):787-90.
Hunter T. Oncogenes and proto-oncogenes: how do they differ? J Natl Cancer Inst. 1984 Oct;73(4):773-86. PMID: 6434779.
Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;2004(26):2704-14.
Ito Y, Kobayashi S, Taniguchi T, et al. Frequent detection of K-ras mutation in stool samples of colorectal carcinoma patients after improved DNA extraction: comparison with tissue samples. International Journal of Oncology. 2002 Jun;20(6):1263-68.
Itzkowitz SH, Jandorf L, Brand R, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007 Jan;5(1):111-17.
Jin YM, Li BJ, Qu B, et al. BRAF, K-ras and BAT26 mutations in colorectal polyps and stool. World J Gastroenterol. 2006 Aug 28;12(32):5148-52.
Lee JK, L.E. Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis. Ann Intern Med. 2014;160(3):171-81.
Lidgard GP, Domanico MJ, Bruinsma JJ et al. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013 April 29.
Olson J, Whitney DH, Durkee K, et al. DNA stabilization is critical for maximizing performance of fecal DNA-based colorectal cancer tests. Diagn Mol Pathol. 2005 Sep;14(3):183-91.
Onouchi S, Matsushita H, Moriya Y, et al. New method for colorectal cancer diagnosis based on SSCP analysis of DNA from exfoliated colonocytes in naturally evacuated feces. Anticancer Research. 2008 Jan;28(1A):145-50.
Onouchi S, Matsushita H, Nomura S, et al. PCR-based assessment of the recovery rate of exfoliated colonocytes or cancer cells from fecal samples depends on the storage conditions after defecation. J Gastrointestinal & Liver Diseases. 2007 Dec;16(4):369-72.
Melotte V, Lentjes MH, van den Bosch SM, Hellebrekers DM, de Hoon JP, Wouters KA, Daenen KL, Partouns-Hendriks IE, Stessels F, Louwagie J, Smits KM, Weijenberg MP, Sanduleanu S, Khalid-de Bakker CA, Oort FA, Meijer GA, Jonkers DM, Herman JG, de Bruïne AP, van Engeland M. N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst. 2009 Jul 1;101(13):916-27. Epub 2009 Jun 17.
Nishikawa T, Maemura K, Hirata I, et al. A simple method of detecting K-ras point mutations in stool samples for colorectal cancer screening using one-step polymerase chain reaction/restriction fragment length polymorphism analysis. Clinica Chimica Acta. 2002 Apr;318(1-2):107-12.
Syngal S, Stoffel E, Chung D, et al. Detection of stool DNA mutations before and after treatment of colorectal neoplasia. Cancer. 2006 Jan 15;106(2):277-83.
Tagore KS, Lawson MJ, Yucaitis JA, et al. Sensitivity and specificity of a stool DNA multitarget assay panel for the detection of advanced colorectal neoplasia. Clin Colorectal Cancer. 2003 May;3(1):47-53.
Wan J, Zhang ZQ, You WD, et al. Detection of K-ras gene mutation in fecal samples from elderly large intestinal cancer patients and its diagnostic significance. World J Gastroenterol. 2004 Mar 1;10(5):743-46.
Whitney D, Skoletsky J, Moore K, et al. Enhanced retrieval of DNA from human fecal samples results in improved performance of colorectal cancer screening test. J Mol Diagn. 2004 Nov;6(4):386-95.
Zou H, Allawi H, Cao X, et al. Quantification of methylated markers with a multiplex methylation-specific technology. Clin Chem 2012;58(2):375-383.


