We are proposing changes to the 20.4 NCD that reflect the 2005 reconsideration as described below:
All other indications for ICDs not currently covered in accordance with this decision may be covered under Category B IDE trials (42 CFR 405.201).
See Appendix B for the proposed manual language.
CMS is seeking comments on our proposed decision. We will respond to public comments in a final decision memorandum, as required by §1862(l)(3) of the Social Security Act (the Act).
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology:
CMS initiated this national coverage determination (NCD) to consider coverage under the Medicare Program for implantable cardioverter defibrillators (ICDs; often referred to as “defibrillators”).
The scope of this review is limited to ICDs. While we reference cardiac resynchronization therapy defibrillator (CRT-D) devices in this document since these devices have defibrillator functions, CRT devices are outside the scope of this decision. CRT devices are currently covered at local contractor discretion and not currently subject to an NCD.
Sudden cardiac death (SCD) is defined as the sudden and unexpected death from cardiac causes within an hour of the onset of symptoms (Bonow, Mann, Zipes, & Libby, 2012). Heart disease is the leading cause of death in the US and accounts for approximately 24% of all deaths, and SCD in turn is estimated to be the final cause of approximately 50% of all cardiac deaths (Centers for Disease Control and Prevention [CDC], 2017; Hayashi, Shimizu, & Albert, 2015). Stecker et al. (2014) summarize the “rule of 50’s” for SCD: it accounts for up to 50% of all cardiac deaths; 50% of the SCD’s are the first cardiac event; and SCD accounts for up to 50% of potentially productive years of life lost due to premature death or disabilities.
Sudden cardiac arrest is defined as the “sudden cessation of effective cardiac mechanical activity resulting in unresponsiveness, without normal breathing or signs of circulation” (Russo et al., 2013). If not resuscitated, patients with cardiac arrest will progress to sudden cardiac death. Sudden cardiac arrest and death in turn are most often associated with life-threatening ventricular tachyarrhythmias (rapid heart rates arising from the ventricles): sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). Sustained VT may degenerate into VF and then asystole (in which all cardiac electrical activity stops, and the heart stops beating).
Sustained VT is defined as VT lasting ≥30 seconds or terminated by medical intervention (either cardioversion or pacing) before that time (Russo et al., 2013). While tachycardia is defined technically as a heart rate of ≥100 beats per minute, many experts believe that in hemodynamically unstable patients, “if the heart rate is <150 beats per minute, it is unlikely that the symptoms of instability are caused primarily by the tachycardia unless there is impaired ventricular function” (i.e., a depressed left ventricular ejection fraction). Thus, “a heart rate >150 beats per minute is usually an inappropriate response to physiological stress (e.g., fever, dehydration) or other underlying conditions” and in unstable patients, this higher heart rate is likely a major contributor or the primary cause of the symptoms of instability (American Heart Association, 2016a; American Heart Association, 2016b).
In cardiomyopathy, the heart muscle becomes enlarged, thick, or rigid. In the vast majority of cases, the underlying cardiomyopathy that increases SCD risk is due to ischemia (coronary artery disease causing blockages of the arteries which reduces blood flow and oxygen to the heart muscle, which in turn both decreases the heart’s ability to pump blood and oxygen to the vital organs (e.g., brain, kidneys), and increases the risk of life-threatening cardiac
arrhythmias); we refer to this as ischemic cardiomyopathy.
The underlying cardiomyopathy may also be due to non-ischemic causes, such as infection, inflammation, familial or genetic conditions or idiopathic causes; we generally refer to this as non-ischemic cardiomyopathy, although in this document we break out familial or genetic etiologies into a separate SCD primary prevention risk category. The two major categories of cardiomyopathy – ischemic and non-ischemic – are not mutually exclusive; some patients may have elements of both.
Heart failure can affect the right side of the heart only, or it can affect both sides of the heart. Most cases involve both sides of the heart and in turn is a clinical diagnosis that is associated with a depressed left ventricular ejection fraction (LVEF) as measured by diagnostic imaging studies (echocardiography, radionuclide scanning, cardiac magnetic resonance imaging (MRI), or catheter angiography). Most trials on prevention of sudden cardiac death have used a LVEF ≤35% in their inclusion criteria. The New York Heart Association (NYHA) Functional Classification is the most commonly used classification system in both clinical practice and trial inclusion criteria (see Appendix D). It classifies the severity of heart failure in terms of symptoms (such as shortness of breath) and limitations in physical activity (e.g., Class I patients have no symptoms or limitations with ordinary activity; Class IV patients have symptoms at rest, and severe limitations in physical activity).
An ICD is a battery-driven electronic device placed in the chest, and connected to the heart by leads. The device monitors the heart’s electrical activity, detects the onset of life-threatening tachyarrhythmias, and tries to terminate these first by smaller electrical stimuli, and then with shock therapy. The “shock” is a defibrillation – a delivery of an adequate amount of electrical current with the goal of temporarily stopping all cardiac electrical signals (essentially producing a temporary state of asystole), which then allows the heart to “reset” itself back to a normal sinus rhythm.
These shocks can be effective in terminating tachyarrhythmias but can be quite painful and disruptive to the patient. Shocks are said to be “appropriate” if they are in response to true, life-threatening tachyarrhythmias; “inappropriate” shocks are those triggered by arrhythmias that are not potentially life-threatening. Inappropriate shocks can also be triggered by malfunction of the device/leads. Inappropriate shocks have been reported in 6%-20% of patients, and in 5.9% of patients in the 2016 DANISH trial (Køber et al., 2016; Priori et al., 2015; Providência et al., 2015; van der Heijden et al., 2015). At the same time, defibrillators never actually “fire” (no therapeutic shocks are delivered) in the majority of patients who receive them for primary prevention (Merchant, Quest, Leon, & El-Chami, 2016; Priori et al., 2015).
ICDs can only treat arrest and life-threatening ventricular tachyarrhythmias, and thus their ability to reduce all-cause mortality (i.e., improve patient survivability) depends on a relatively high frequency of arrhythmic deaths compared to death by other causes, for any given population being treated with ICDs.
Patients with ICDs [whether alone or combined with a cardiac resynchronization therapy device (CRT)] require constant physician follow up typically for the rest of their lives, to include regular device interrogations every 3-6 months. Adverse events can include, in addition to inappropriate shocks, procedure-related pneumothorax (collection of air in the chest cavity which causes the lung to collapse) and cardiac tamponade (pressure on the heart that occurs due to the accumulation of fluid in the pericardial space), and device-related infections – all of which are potentially life-threatening. Device infections have been reported previously in 1-7% of patients, and the more recent DANISH trial observed rates of 4.7% in the CRT-D group and 5.1% in the ICD group (Køber et al, 2016; Mulpuru, Pretorius, & Birgersdotter-Green, 2013; van der Heijden, 2015). In addition to being life-threatening, such adverse events require further treatment, hospitalizations, imaging and procedures. When battery life expires (typically within 5-7 years) a procedural replacement of the device itself is necessary. Patients with defibrillators often live with anxiety and depressive symptoms due to fear of either a shock or device failure (Freedenberg, Thomas, & Friedmann, 2011). As with any medical intervention, the question of whether the demonstrated benefits outweigh the harms for particular patients persists.
CMS issued an NCD in 1986 providing limited coverage of implantable defibrillators. The policy has expanded over the years with revisions in 1991, 1999, 2003, 2004, and 2005. In the June 6, 2003 decision memorandum, coverage was expanded to patients with a previous myocardial infarction, low ejection fraction and a wide QRS interval. The policy was also expanded to include coverage to patients enrolled in an Investigational Device Exemption Category B device trial. A follow up decision memorandum to clarify this specific aspect of the policy was published March 12, 2004.
On March 30, 2004, CMS accepted a request from Medtronic Inc. to expand coverage for ICDs. Medtronic Inc. made this request based on the results of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) and specifically requested that Medicare expand coverage to the trial population. On December 16, 2004, CMS announced its concern on the absence of publication of the SCD-HeFT data and the potential for closing the NCD. Publication did not occur prior to the December 28, 2004 deadline so on that day CMS posted a final DM that continued current coverage. CMS opened a reconsideration of that decision on December 29, 2004 in anticipation of the SCD-HeFT publication, and a decision based on SCD-HeFT data was finalized on January 27, 2005.
Since there is an existing NCD for ICDs, this review is a reconsideration of the current policy. The current policy is codified in 20.4 of the Medicare National Coverage Determinations manual. Section 20.4 of the NCD Manual has been included at Appendix C.
CMS opened this NCA to reconsider coverage indications for ICDs.
Medicare is a defined benefit program. For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Social Security Act.
The FDA approved the first implantable defibrillator in 1985 while the first implantable cardioverter defibrillators were approved in 1988 and 1989. The FDA approves each device individually and has granted premarket approvals (PMA) for implantable defibrillators for the indications of providing antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias.
On September 28, 2012, the FDA approved the first subcutaneous implantable defibrillator, the EMBLEM S-ICD system by Boston Scientific. This device is indicated to provide defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia, incessant ventricular tachycardia, or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing.
When making national coverage determinations, CMS generally evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. The critical appraisal of the evidence enables us to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for beneficiaries. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
A detailed account of the methodological principles of study design that the Agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A.
Public comments sometimes cite published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information will be redacted or will not be made available on the CMS website CMS responds in detail to the public comments on a proposed national coverage determination when issuing the final national coverage determination.
CMS last reconsidered the ICD NCD (see Appendix C for § 20.4 of the NCD) in January of 2005. CMS has opened this national coverage analysis to reconsider coverage indications for ICDs based on more recent scientific evidence and will discuss in this section relevant evidence established after that date.
For this reconsideration, we reviewed the published medical literature since 2005 to 2017 to determine reasonable and necessary indications for ICDs and whether the data collection questions have been answered.
Our review and analysis of the evidence on the clinical utility of ICDs in symptomatic patients who have not experienced a prior episode of cardiac arrest or sustained ventricular tachyarrhythmias (sustained ventricular tachycardia or ventricular fibrillation), and thus whether ICDs are reasonable and necessary to treat certain Medicare patients, is guided by the following questions:
Is there evidence to conclude that ICDs decrease mortality for patients with non-ischemic dilated cardiomyopathy (NIDCM) and reduced LVEF?
2. External Technology Assessments
While CMS did not request an external technology assessment (TA) as part of this reconsideration, a TA had been requested prior to the opening of this NCA (2013) that assessed the effectiveness of defibrillators.
Uhlig K, Balk E, Earley A, et al. Assessment on implantable defibrillators and the evidence for primary prevention of sudden cardiac death. (Prepared by the Tufts Evidence-based Practice Center under Contract No. HHSA 290-2007-10055-I) Rockville, MD: Agency for Healthcare Research and Quality. June 2013.
The authors, funded by the US Agency for Healthcare Research and Quality, performed a methodical technology assessment to include a systematic literature review and meta-analysis to assess the clinical effectiveness of ICD use for primary prevention of sudden cardiac death. The assessment focused on three key questions, examining:
- ICD versus no ICD, ICD with antitachycardia pacing versus ICD alone, or ICD with CRT versus ICD alone, and differences among subgroups;
- Early and late adverse events and inappropriate shocks after ICD implantation, and differences among subgroups; and
- Eligibility criteria and evaluation methods for patients included in comparative studies and the risk of SCD.
The assessment did not examine the possible added benefit of ICD in
patients who are to receive CRT (i.e., CRT-D v. CRT-P).
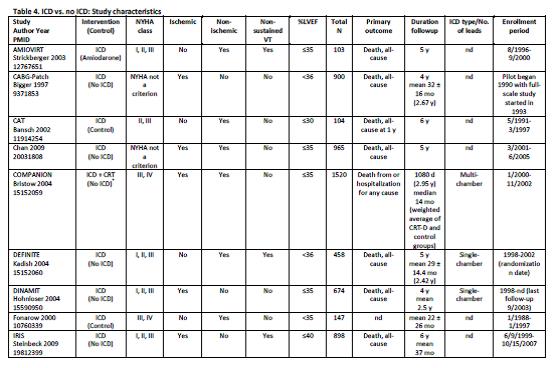
Uhlig et al. Assessment on Implantable Defibrillators and the Evidence for Primary Prevention of Sudden Cardiac Death. 2013. Table 4, Page 26.
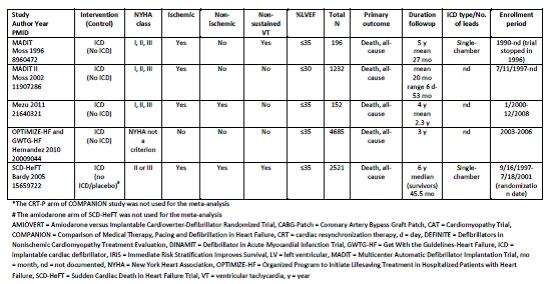
Uhlig et al. Assessment on Implantable Defibrillators and the Evidence for Primary Prevention of Sudden Cardiac Death. 2013. Table 4, Page 27.
Uhlig and colleagues concluded that “there is a high strength of evidence that ICD therapy for primary prevention of SCD, versus no ICD therapy, shows benefit with regard to all-cause mortality and SCD in patients with reduced left ventricular ejection fraction and ischemic or non-ischemic cardiomyopathy beyond the immediate post-MI or coronary revascularization periods. Studies failed to show statistically significant differences for all-cause mortality across subgroups. There is insufficient evidence for all-cause mortality for patients who receive CRT-Ds versus ICD alone for primary prevention. There is high strength of evidence that in-hospital adverse events are infrequent (1-3%) and moderate strength of evidence that up to one-fifth of patients receive inappropriate shocks from the ICDs.”
3. Internal Technology Assessment
Literature Search Methods
CMS searched Pubmed MEDLINE, Embase, ClinicalTrials.gov, and the Cochrane Central Registry of Controlled Trials, from 2004 through May 2017 (ensuring overlap of the literature reviewed in the 2005 NCD). Search keywords included combinations of: implantable cardioverter defibrillator, ICD, defibrillator, sudden cardiac death, ventricular arrhythmias, ischemic cardiomyopathy, non-ischemic cardiomyopathy, randomized, and cardiac resynchronization therapy. This evidence review primarily focuses on randomized controlled trials that assess the clinical utility of defibrillators compared to optimal medical therapy, and relevant formal Technology Assessments and professional society guidelines. In our analysis we reference other trials, observational studies and analyses, as well as relevant published correspondence and editorials, as these can assist both in interpreting these trials and identifying evidence gaps. Abstracts only and publications in languages other than English were excluded. Trials on CRT only were also excluded.
We reviewed all original research that is detailed in the ACC listing of published manuscripts from the ACC NCDR ICD Registry. We additionally performed a Pubmed search using the terms ICD or defibrillator and NCDR (National Cardiovascular Data Registry) focusing on all (including observational) studies, to assess the extent to which the published literature has addressed the ten “initial hypotheses” for this registry as stated in our 2005 NCD.
Technology Assessments
Colquitt J, Mendes D, Clegg A, et al. Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronization therapy for the treatment of heart failure: Systematic review and economic evaluation. Health Technol Assess 2014;18(56)
The authors, funded by the UK National Institute for Health Research, performed a methodical technical assessment to include a systematic literature review and meta-analysis to assess:
- The clinical effectiveness of ICDs for people at increased risk of sudden cardiac death as a result of ventricular arrhythmias despite receiving optimal pharmacological therapy;
- CRT with or without a defibrillator (CRT-D v. CRT-P) in addition to optimal pharmacological therapy for people with heart failure as a result of left ventricular systolic dysfunction (LVSD) and cardiac dyssynchrony; and
- CRT-D in addition to optimal pharmacological therapy for people with both conditions above.
Studies

Colquitt et al. Health Technol Assess. 2014 Aug;18(56):1-560. Table 6, Page 20.
As to the clinical effectiveness of ICDs, Colquitt and colleagues found that “ICDs reduced all-cause mortality in people at increased risk of SCD, defined in trials as those with previous ventricular arrhythmias/cardiac arrest, myocardial infarction (MI) > 3 weeks previously, non-ischemic cardiomyopathy (depending on data included) or ischemic/non-ischemic heart failure (HF) and left ventricular ejection fraction ≤ 35%. There was no benefit in people scheduled for coronary artery bypass graft. A reduction in SCD but not all-cause mortality was found in people with recent MI. CRT-P and CRT-D reduced mortality and HF hospitalizations, and improved other outcomes, in people with HF as a result of LVSD and cardiac dyssynchrony when compared with optimal pharmacological therapy (OPT). In people with both conditions, CRT-D reduced the risk of all-cause mortality and HF hospitalization, and improved other outcomes, compared with ICDs.”
The authors also concluded that “an RCT comparing CRT-D and CRT-P in people with HF as a result of LVSD and cardiac dyssynchrony is required, for both those with and those without an ICD indication. A RCT is also needed into the benefits of ICD in non-ischemic cardiomyopathy in the absence of dyssynchrony.”
Meta-Analyses
Al-Khatib S, Fonarow G, Joglar J, et al. Primary prevention implantable cardioverter defibrillators in patients with nonischemic cardiomyopathy: A meta-analysis. JAMA Cardiol. 2017;2(6):685-688.
The purpose of this meta-analysis was to “investigate the association of primary prevention ICDs with all-cause mortality in patients with nonischemic cardiomyopathy.” The authors pooled data from four trials: DEFINITE (2004), CAT (2002), SCD-HeFT (2005) and DANISH (2016); please see the Evidence Table (Figure 1) for summaries of the trials. A fifth trial (and one of four that examined non-ischemic cardiomyopathy patients only), AMIOVIRT (2003), was excluded because the trial compared ICD to an antiarrhythmic medication, amiodarone, rather than to standard optimal medical therapy. The authors included the non-ischemic patient subgroup from SCD-HeFT (the sole trial that included both ischemic and non-ischemic cardiomyopathy patients), and the DANISH subgroup of patients who did not receive a CRT (as CRT patients were excluded generally from the analysis).
Pooling these selected patients from these four trials, and using fixed- and random-effects models, the authors showed that use of an ICD significantly reduced all-cause mortality (HR 0.75; CI 0.61-0.93; P=.008, P=0.87 for heterogeneity). The authors concluded that these findings supported the 2012 AHA/ACCF/HRS guidelines recommending the use of ICDs in certain patients with non-ischemic cardiomyopathy and depressed LVEF.
Golwala H, Bajaj N, Arora G, et al. Implantable cardioverter-defibrillator for nonischemic cardiomyopathy: An updated meta-analysis. Circulation. 2017;135:201–203.
This meta-analysis also assessed the association of ICDs for primary prevention with all-cause mortality in patients with non-ischemic cardiomyopathy. The authors pooled data from non-ischemic patients from six trials: CAT (2002), AMIOVIRT (2003), DEFINITE (2004), SCD-HeFT (2016), COMPANION (2004), and DANISH (2016). The authors did three separate analyses: combining all of the trials; combining ICD patients only, thus excluding CRT patients, for the five relevant trials; and combining CRT patients only, from the two relevant trials (DANISH and COMPANION). (See the Evidence Table, Figure 1, for summaries of these trials.)
For the first analysis, pooling all six trials, the authors showed that use of an ICD significantly reduced all-cause mortality risk by 23% (HR 0.77; CI 0.64–0.91). The second pooled analysis assessing ICD plus optimal medical therapy versus optimal medical therapy alone showed that use of an ICD significantly reduced all-cause mortality risk by 24% (HR 0.76; CI, 0.62–0.94). The third pooled analysis assessing ICD plus CRT (CRT-D) plus optimal medical therapy versus CRT plus optimal medical therapy alone, showed no significant difference between the two groups (HR 0.70; CI 0.39–1.26); thus no survival benefit when ICD is used in patients in addition to CRT. (P values were not reported.)
The authors concluded that “this incremental reduction of all-cause mortality with ICD is substantial and provides support to the existing [ACC/AHA/HRS] guidelines until we acquire additional data. . . Furthermore, adequately powered randomized studies are needed before recommending any change in existing guidelines . . .”
Randomized Controlled Trials
This section summarizes the randomized controlled trials since the 2005 NCD that assess the clinical utility of defibrillators in the primary prevention of sudden cardiac death. The evidence table (Figure 1) summarizes all major relevant randomized controlled trials; the trials since the 2005 NCD are in italics.
Figure 1: Major Randomized Controlled Trials Assessing Defibrillators for Primary Prevention of Sudden Cardiac Death
| Year |
Trial / Author(s) |
Population |
Intervention/Comparator |
Outcome |
Time (mos) |
Result |
| |
Ischemic CM |
|
|
|
|
|
1996 |
MADIT / Moss et al. |
n = 196
NYHA 1-2
EF ≤35%
Post MI 3 wks
NSVT
EP induced VT |
ICD v AA drug (mostly amiodarone) |
ACM |
27m |
HR 0.46 (95% CI, 0.26-0.82) |
| 1997 |
CABG Patch (post CABG) / Bigger |
n = 900
NYHA 1-3
EF ≤35%
Sched for CABG
Abnl SAECG |
ICD v. OMT
(OMT = drugs plus revascular.) |
ACM |
32m |
HR 1.07 (95% CI, 0.81-1.42) |
| 2002 |
MADIT II / Moss et al. |
n = 1,232
NYHA 1-3
EF ≤30%
Post-MI ≥1m, ≥3m if CABG |
ICD v OPT |
ACM |
20m |
HR 0.69 (95% CI, 0.51-0.93) |
| 2004 |
DINAMIT (early post-MI) / Hohnloser et al. |
n = 674
NYHA 1-3
EF ≤35%
Post-MI (6-40 days, mean 18)
Abnl HR |
ICD v OPT |
ACM |
33m |
HR 1.08 (95% CI, 0.76-1.55) |
| 2009 |
IRIS (early post-MI) / Steinbeck et al. |
n = 898
Post-MI (5-31 days) AND one of:
a. EF ≤40%, HR ≥90bpm
b. rapid NSVT
c. both a and b |
ICD v OPT |
ACM |
37m |
HR 1.04 (95% CI, 0.81-1.35) |
| |
Both Ischemic and Non-ischemic CM |
|
|
|
|
|
| 2004 |
COMPANION / Bristow et al. |
n = 1,520
(ICM = 838 NICM = 682)
NYHA 3-4
EF ≤35%
QRS ≥120 msec |
1:2:2
OPT : CRT-P : CRT-D |
ACM or Hosp (all) |
12m : 16m : 16m |
All pts:
- POS: CRT-P (v OPT), HR 0.81 (95% CI, 0.69-0.96)
- POS: CRT-D (v OPT), HR 0.80 (95% CI, 0.68-0.95)
2nd (ACM alone):
- NEG: CRT-P, HR 0.76 (95% CI, 0.58-1.01)
- POS CRT-D, HR 0.64 (95% CI, 0.48-0.86) |
| 2005 |
SCD-HeFT / Bardy et al. |
n = 2,521
(ICM = 52% NICM = 48%)
NYHA 2-3
EF ≤35%
OPT for 3m prior to R |
1:1:1
ICD : amiodarone : placebo
(baseline OPT for all) |
ACM |
46m |
POS: ICD v placebo, HR 0.73 (95% CI, 0.52-1.02) |
| |
Non-ischemic CM |
|
|
|
|
|
| 2002 |
CAT / Bansch et al. |
n = 104
NYHA 2-3
EF ≤30%
New onset CM (≤9m) |
ICD v OPT |
ACM |
66m |
HR 0.81 (95% CI, 0.38-1.71) |
| 2003 |
AMIOVIRT / Strickberger et al. |
n = 103
NYHA 1-3
EF ≤35%
Asymptomatic NSVT |
ICD v. amiodarone
[?baseline OPT] |
ACM |
36m |
HR 0.87 (95% CI, 0.31-2.42) |
| 2004 |
DEFINITE / Kadish et al. |
n = 458
NYHA 1-3
EF ≤35%
NSVT |
ICD v OPT |
ACM |
29m |
HR 0.65 (95% CI, 0.40-1.06) |
| 2016 |
DANISH / Køber et al. |
n = 1116
NYHA 2-3 (or 4 if CRT planned)
EF ≤35%
NT-proBNP >200 pg/mL |
ICD or CRT-D + OPT v OMT alone (OPT + CRT if needed) |
ACM |
68m |
CRT:
HR 0.91 (95% CI: 0.64-1.29)
No CRT:
HR 0.83 (95% CI: 0.58-1.19) |
| |
|
|
|
|
|
|
NYHA: New York Heart Association. MI: Myocardial infarction. NSVT: Non-sustained ventricular tachycardia. EP: Electrophysiological study. VT: Ventricular tachycardia. AA: Anti-arrhythmic drug.
Note that the Multicenter Unsustained Tachycardia Trial (MUSTT), which was initiated in 1989 and reported in 1999, is not included in the analysis since it did not randomize to drug therapy or ICD (Buxton et al., 1999). The 2014 Technology Assessment by Colquitt et al. explicitly excluded this trial (for further critique of that trial, see Colquitt et al., 2014).
Steinbeck G., M.D., Andresen D., Seidl K., et al, for the IRIS Investigators. Defibrillator implantation early after myocardial infarction. NEJM 2009;Oct 8; 361(15):1427-36.
This outside the United States multicenter, open-label, randomized controlled trial randomized patients with acute MI with or without ST-segment elevation to either ICD plus OMT or individualized OMT alone. It assessed whether there is a survival benefit from ICD selected, asymptomatic survivors of acute MI. Randomization was stratified to ensure a balanced number of patients with ST elevated and non-ST elevated myocardial infarction between the ICD and control groups. The primary outcome was time to all-cause mortality post-acute-MI and major secondary outcomes included SCD, non-SCD, and noncardiac deaths.
Patients needed to have one of the following criteria: 1) a heart rate of 90 beats per minute or more on the first available ECG (obtained within 48 hours after the MI) and an LVEF < 40% or (on one of days 5 to 31 after MI) and/or 2) nonsustained VT consisting of three or more consecutive ventricular premature beats during Holter ECG monitoring, with a heart rate of 150 beats per minute or more (on days 5 to 31). Participants were excluded if they had ventricular arrhythmias that occurred before the index infarction or more than 48 hours after the event and that required treatment, NYHA class IV drug-refractory heart failure, an interval of more than 31 days between myocardial infarction and presentation, no ECG documentation within the first 48 hours after the onset of chest pain, an indication for coronary-artery bypass (CABG) surgery before study entry.
A total of 898 patients (86% of whom were still in the hospital) were randomized (445 ICD and 453 to receive medical therapy alone at a mean (±SD) of 13±7 days after infarction.
Over a period of approximately eight years (1999 – 2007), 898 patients were enrolled across 80 hospitals throughout Germany and other European countries. Greater than 90% of patients were discharged on appropriate post-MI medications. Patients were followed for 37 months on average. The two groups were balanced in terms of baseline characteristics, with the exception that diabetes and left bundle-branch block were slightly more frequent in the ICD group (P=0.03 and P=0.05, respectively).
Prophylactic ICD therapy did not reduce all-cause mortality (hazard ratio [HR], 1.04; 95% confidence interval [CI], 0.81 to 1.35; P=0.78). There were fewer SCDs in the ICD group than in the control group (27 vs. 60; HR, 0.55; 95% CI, 0.31 to 1.00; P=0.049), but the number of non-SCDs was higher (68 vs. 39; HR, 1.92; 95% CI, 1.29 to 2.84; P=0.001). The risk of SCD was reduced with ICD therapy, however this was offset by an increase in the risk of non-SCD. Prespecified subgroup analysis suggested that the results did not depend on which of the enrollment criteria patients met.
Køber L., Thune J., Nielsen J., Haarbo J., et al, for he DANISH Investigators. Defibrillator implantation in patients with nonischemic systolic heart failure. NEJM 2016; Sep 29; 375(13):1221-30.
The DANISH study was an open-label, randomized, controlled, multicenter study to assess the effect of ICDs in patients with non-ischemic systolic HF on mortality. Randomization was stratified by center and by patients scheduled to receive CRT, to ensure balance between control and intervention arms. This primary prevention trial included symptomatic patients (NYHA class II or III, or NYHA class IV if CRT was planned) with non-ischemic systolic heart failure (left ventricular ejection fraction ≤35%, not due to coronary heart disease) and an increased level (>200 pg per milliliter) of N-terminal pro–brain natriuretic peptide (NT-proBNP). Major exclusion criteria were permanent atrial fibrillation with a resting heart rate higher than 100 beats per minute or renal failure that was being treated with dialysis. The primary outcome was time to all-cause mortality. Secondary outcomes were cardiac death, SCD, resuscitated cardiac arrest or sustained VT, and change from baseline in quality of life. Prespecified subgroup analyses included age and CRT status.
Over a period of approximately six years (2008 – 2014), a total of 1,116 participants were enrolled across five centers in Denmark with 471 in the no CRT stratum (234 ICD and 237 OPT) and 645 in the CRT stratum (322 CRT-D and 323 CRT-P) with 58% of the total population receiving a CRT. In the CRT stratum, the two groups were balanced in terms of baseline characteristics. In the no CRT stratum, the ICD group had a substantially higher baseline NT-proBNP (1,277
versus 862 (pg/mL).
The population, with a median age 64 years (interquartile range 56-72 years), were followed for a median of 67.6 months (interquartile range, 49 to 85). For optimal pharmacologic therapy, almost all patients received beta-blockers and inhibitors of the renin-angiotensin system and 60% received mineralocorticoid-receptor antagonists.
The trial found that ICD implantation for primary prevention in patients with symptomatic non-ischemic HF did not provide an overall survival benefit (the primary outcome) compared to usual clinical care (HR 0.87; 95% CI 0.68 to 1.12; P=0.28). Neither was there a benefit for the secondary outcome of all CV deaths (HR 0.77, 95% CI 0.57 to 1.05, P=0.10). However, the risk of SCD specifically (also a secondary outcome) was halved with an ICD (HR 0.5; 95% CI 0.31 to 0.82; P=0.005). The results were independent of whether a patient received a CRT device (there was no significant interaction in the subgroup analysis).
When combining the CRT and no CRT strata, the prespecified subgroup analysis suggested that younger patients (<59 years) may have an overall survival benefit with an ICD (HR 0.51, CI 0.29-0.92, p=0.02).
While the model p-value for NT-proBNP was p=0.06, the stratum specific HR for <1177 pg/ml (HR, 0.59, 95% CI 0.38−0.91, p=0.02) supporting a benefit with ICD in this group. No other prespecified subgroup analysis demonstrated notable treatment-by-subgroup interaction.
Observational Studies Using the NCDR
The eight studies reviewed below all used the NCDR prominently in their analyses. These studies were not specifically designed to target a particular “initial hypothesis” for the registry as identified by CMS in 2005; nor were protocols for these studies submitted to or approved by CMS. However, we believe the research questions for each of these studies below are related to one or more of these ten hypotheses; and collectively, these eight studies are related to all ten hypotheses. We are aware that there are numerous other published studies that may be related to one or more of these hypotheses, or aspects of them.
We add the ten “initial hypotheses” for the registry again below for readers’ convenience.
Initial hypotheses that were to be addressed by the NCDR database included the following:
- The clinical characteristics of the patients receiving ICDs are similar to those of patients involved in the primary prevention randomized clinical trials.
- The indications for ICD implantation in patients are similar to those in the primary prevention randomized clinical trials.
- The in-hospital procedure related complications for patients are similar to those in the primary prevention randomized clinical trials.
- Certified providers competent in ICD implantation are implanting ICD devices in patients.
- Patients who receive an ICD represent patients for which current clinical guidelines and the evidence base recommend implantation.
- The clinical characteristics and indications for ICD implantation do not differ significantly among facilities.
- The clinical characteristics and indications for ICD implantation do not differ significantly among providers.
- The in-hospital procedure related complications for ICD implantation do not differ significantly among facilities.
- The in-hospital procedure related complications for ICD implantation do not differ significantly among providers.
- The in-hospital procedure related complications for ICD implantation do not differ significantly among device manufacturer, types, and/or programming
Al-Khatib SM1, Hellkamp A, Bardy GH. et al. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials JAMA. 2013 Jan 2;309(1):55-62.
This is a retrospective analysis comparing patients from the NCDR ICD Registry who met MADIT-II or SCD-HeFT study criteria to the respective patients from those clinical trials. The study objective was to determine whether trial-eligible patients receiving a primary prevention ICD have an all-cause mortality rate that differs from the two largest primary prevention clinical trials MADIT-II and SCD-HeFT.
All patients enrolled in MADIT-II (n=1232) and patients randomized to receive placebo or ICD therapy in SCD-HeFT (n=1676) were included. The registry was queried for all patients implanted from January 1, 2006, through December 31, 2007 who had a history of a myocardial infarction and an LVEF 30% or less. Those meeting the MADIT-II criteria (2464 propensity score-matched patients) or the SCD-HeFT criteria (3352 propensity score-matched patients) were included. Comparability of clinical characteristics between the registry and trial populations was assessed for the entire study population. Survival in the registry matched patients was compared to those who received primary prevention ICDs in MADIT-II (n = 742) and SCD-HeFT (n = 829).
Registry patients were substantially older and had a higher burden of comorbidities compared to clinical trial participants. The median follow-up in MADIT-II and the matched registry patients was 19.5 months and 35.8, respectively. The median follow- up in SCD-HeFT and the matched registry patients was 46.1 months and 35.0 months, respectively. There was not statistically significant difference in survival between MADIT-II-like registry patients and MADIT-II ICD patients (2-year mortality rates: 13.9% and 15.6%, respectively. The survival curves for the SCD-HeFT-like registry patients was coincidental with the SCD-HeFT ICD arm (3-year mortality rates: 17.3% and 17.4%, respectively. No survival differences were identified after restricting results to patients ≥ 65 years of age and upon covariate adjustment.
Masoudi FA, Go AS, Magid DJ, et al. Longitudinal study of implantable cardioverter-defibrillators: Methods and clinical characteristics of patients receiving implantable cardioverter-defibrillators for primary prevention in contemporary practice. Circ Cardiovasc Qual Outcomes. 2012 Nov;5(6):e78-85.
This is a retrospective cohort study of patients, January 2006 to December 2009, with a new ICD (no prior ICD) for primary prevention and LVEF <50% implanted at one of 7 health plans of the Health Maintenance Organization Research Network (Henry Ford Health System, Kaiser Permanente [Colorado, Northern California, Northwest Southern California], Marshfield Clinic, and Meyers/Fallon Community Health Plan/U. Mass). The study also includes data on adjudicated arrhythmia episodes resulting in device therapies including shocks and ATP through manual medical record abstraction at the study sites with central clinical review and adjudication of source documentation.
The purpose of this study is to assess the extent to which the clinical characteristics and long-term (~ 3 years) outcomes of registry patients with left ventricular systolic dysfunction undergoing primary prevention ICD implantation differ from those enrolled in the randomized, controlled trials. During the enrollment period, 3254 patients underwent primary prevention ICD placement with a final cohort comprised 2621 patients with LVSD after exclusions. The characteristics of the study cohort was compared to those of the MADIT-II (n=742) and SCD-HeFT (n=849) trials.
The mean age of the registry cohort was higher than that of the populations from the RCTs, ranging from almost 7 years higher than the mean age of the SCD-HeFT population to nearly 3 years higher than the average age of the population of MADIT-II. LVEF in the cohort was 25% (± 6.9%) compared to the RCTs (MADIT-II 23 ± 5%; SCD-HeFT 23.5 ± 7%). The registry population had a substantially higher comorbidity burden, including higher diabetes mellitus, hypertension, and atrial fibrillation/flutter. Registry patients were more likely to receive β blockers and statins and less likely to receive digoxin. Data on ICD therapy and outcomes (mortality and hospitalizations) was still being collected at the time that this manuscript was published.
Freeman JV1, Wang Y, Curtis JP, et al. Physician procedure volume and complications of cardioverter-defibrillator implantation. Circulation. 2012 Jan 3;125(1):57-64.
This is a retrospective cohort study of patients who had an ICD implanted between April 2006 and March 2010. The purpose of the study was examined the relationship between physician annual ICD implantation volume and in-hospital complications. The primary outcome for this study was any adverse event that occurred during the implantation or preceding hospital discharge. Major adverse events were cardiac arrest, cardiac perforation, valve injury, coronary venous dissection, hemothorax, pneumothorax, deep vein thrombosis, transient ischemic attack, stroke, myocardial infarction, pericardial tamponade, and arteriovenous fistula. Between April 2006 and March 2010, 4011 physicians performed 356,515 initial ICD implantations at 1 of 1463 hospitals which qualified for this study.
Physicians were categorized as electrophysiologists, nonelectrophysiologist cardiologists (“cardiologists”), thoracic surgeons (“surgeons”), physicians who met the training standards for ICD implantation promulgated by the Heart Rhythm Society or none of the above. Physician procedure volume was annualized by dividing the total number of ICD implantations a physician performed by the number of years the physician contributed data to the NCDR ICD Registry. Physicians were ranked by their annualized ICD implantation volume and divided them into quartiles of increasing procedure volume for analysis characteristics (number of beds, teaching status, urban location), and finally hospital annual procedure volume and patient clustering by hospital and physician. The authors evaluated whether the relationship between physician ICD procedure volume and adverse events was independent of physician training and hospital volume.
The relationship between physician annual ICD implantation volume and in-hospital complications was assessed using hierarchical logistic regression to adjust for patient characteristics, implanting physician certification, hospital characteristics, hospital annual procedure volume, and the clustering of patients within hospitals and by physician. The authors also repeated the analysis for ICD subtypes: single chamber, dual chamber, and biventricular.
ICD implant related complications occurred in 3.1% (10,994 patients) with 0.39% (1,375 patients) dying from these complications. The rate of adverse events was lower among patients who received a single-chamber ICD (1.9%) than those implanted with a dual-chamber ICD (2.9%). The complication rate decreased with increasing physician procedure volume from 4.6% in the lowest quartile to 2.9% in the highest quartile, and the mortality rate decreased from 0.72% to 0.36%. The relationship between physician procedure volume and decreased complications remained significant after adjusting for patient, physician, and hospital characteristics (OR 1.55 for complications in lowest-volume quartile compared with highest; 95% confidence interval, 1.34-1.79), and was independent of physician specialty and of hospital volume, was consistent across ICD subtypes, and was also evident for in-hospital mortality.
Curtis JP, Luebbert JJ, Wang, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator JAMA. 2009 Apr 22;301(16):1661-70.
The authors conducted a retrospective cohort study of ICD Registry patients implanted, between January 2006 and June 2007, with an ICD without an epicardial lead or prior ICD implant. The objective of the study was to estimate the association of implanting physician certification with in-hospital procedural complication rates following ICD implantation.
The study investigators grouped implant procedures by the certification status of the implanting physician into mutually exclusive categories: electrophysiologists, nonelectrophysiologist cardiologists, thoracic surgeons, and other specialists. The association of physician certification and risk of in-hospital complications was assessed using hierarchical generalized logistic regression to account for clustering of patients within hospitals.
A total of 111,293 ICD implant procedures met the study criteria with 70.9% (78,857) of the procedures being performed by electrophysiologists, 21.9% (24,399) by nonelectrophysiologist cardiologists, 1.7% (1,862) by thoracic surgeons, and 5.5% (6,175) by other specialists.
The characteristics of patients undergoing ICD implantation differed by physician specialty in several respects, notably by age, race, and payer status. Thoracic surgeons had greater mix of Medicare beneficiaries (75%) compared to the other specialties which ranged from 67-70%. The average patient age for thoracic surgeon patients (70.1 years) was approximately two years older than the other specialties. Thoracic surgeons treated a smaller mix of African Americans but higher mix of Hispanic ethnicity when compared to the other specialties.
There were only clinically modest differences in measures of cardiac status such as history of congestive heart failure, NYHA class, prior cardiac arrest, history of atrial fibrillation, history of ventricular fibrillation, diabetes, hypertension, chronic lung disease, cerebrovascular disease, use of revascularization procedures, LVEF, QRS duration, and blood urea nitrogen measurements across physician certification categories.
Compared with patients whose ICD was implanted by electrophysiologists, patients whose ICD was implanted by either nonelectrophysiologist cardiologists or thoracic surgeons were at increased risk of complications in both unadjusted (electrophysiologists, 3.5% [2743/78,857]; nonelectrophysiologist cardiologists, 4.0% [970/24,399]; thoracic surgeons, 5.8% [108/1862]; with adjusted analyses (relative risk [RR] for nonelectrophysiologist cardiologists, 1.11 [95% confidence interval {CI}, 1.01-1.21]; RR for thoracic surgeons, 1.44 [95% CI, 1.15-1.79]).
Kaiser DW, Tsai V, Heidenreich PA, et al. Defibrillator implantations for primary prevention in the United States: Inappropriate care or inadequate documentation: Insights from the National Cardiovascular Data ICD Registry. Heart Rhythm. 2015 Oct;12(10):2086-93.
This retrospective cohort study, January 2006 to December 2008, utilizing the ICD Registry, sought to assess the patient characteristics associated with not meeting the inclusion criteria of the clinical trials that demonstrated the efficacy of primary prevention ICDs.
Of the 333,993 patients identified from the ICD Registry, a total of 150,264 remained after exclusions for sites with incomplete data reporting, secondary prevention, prior syncope, prior tachycardia arrest, sustained VT, and previous ICD placement.
Of the 150,264, 85.7% (128,821) met inclusion criteria for at least one of the primary prevention trials 77.9% met SCD-HeFT criteria, 39.0% met MADIT II criteria, 1.6% met MUSTT criteria, and 0.9% met MADIT I criteria. On average, patients with older age (> 65 years), prior percutaneous coronary intervention, and prior coronary artery bypass grafting were more likely to meet trial criteria. For non-CRT ICDs, the proportion of implantations that did not meet trial inclusion criteria increased as patient age decreased, from 18% in those > 65 years old to 36% in the age group < 35 years old. In multivariate analysis, the significant predictors for not meeting trial criteria included prior cardiac transplantation (odds ratio [OR] 2.1), pediatric electrophysiology operator (OR 2.0), and high-grade atrioventricular conduction disease (OR 1.4).
Borne R, Peterson P, Greenlee R, et al. Temporal trends in patient characteristics and outcomes among Medicare beneficiaries undergoing primary prevention implantable cardioverter-defibrillator placement in the United States, 2006-2010. Results from the National Cardiovascular Data Registry's Implantable Cardioverter-Defibrillator Registry. Circulation. 2014 Sep 2;130(10):845-53.
The authors performed multivariable hierarchical logistic regression to assess temporal trends in patient characteristics and outcomes among older patients undergoing primary prevention ICD therapy in US hospitals between 2006 and 2010. The cohort included 117,100 patients from the NCDR: Medicare fee-for-service beneficiaries aged ≥65 years and older with LVEF ≤35% who underwent primary prevention ICD implantation, including those receiving concomitant CRT between 2006 and 2010, and could be matched to Medicare claims. Outcomes were all-cause mortality, heart failure hospitalization at 180 days, and device-related complications.
Study results demonstrated that between 2006 and 2010 there were only modest changes in baseline patient clinical characteristics. In the same timeframe, fewer single lead devices and more cardiac resynchronization therapy devices were used over time. There were statistically significant improvements in all outcomes, including 6-month all-cause mortality (7.1% in 2006, 6.5% 2010; adjusted odds ratio 0.88; 95% CI 0.82-0.95), 6-month rehospitalization (36.3% in 2006, 33.7% in 2010; adjusted odds ratio 0.87; 95% CI 0.83-0.91), and device-related complications (5.8% in 2006, 4.8% in 2010; adjusted odds ratio 0.80; 95% CI 0.74-0.88). The authors concluded that, given relatively stable clinical characteristics of patients selected for ICD implantation over this time period, the simultaneous improvements in outcomes “suggest meaningful advances in the care for this patient population.”
Freeman J, Wang Y, Curtis J, et al. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2010 Sep 28;56(14):1133-9.
The authors performed multivariable hierarchical logistic regression to assess the relationship between hospital ICD implantation volume and procedural complications. The study included 224,233 patients from 1,201 US hospitals participating in the NCDR who had an initial ICD implantation between January 2006 and December 2008 and met study exclusion criteria. The primary outcome was any adverse event that occurred in hospital (to include during the implantation procedure). The study accounted for a wide range of patient demographics, clinical history and risk factors, ICD types (single chamber, dual chamber, biventricular) and data, implanting physician certification, and hospital characteristics such as size (patient beds), geographic location (rural, suburban, urban), and type (private/community, government, university).
Study results demonstrated that “the rate of adverse events declined progressively with increasing procedure volume (p trend < 0.0001). This relationship remained significant (p trend < 0.0001) after adjustment for patient clinical characteristics, operator characteristics, and hospital characteristics. The volume-outcome relationship was evident for all ICD subtypes, including single-chamber (p trend = 0.004), dual-chamber (p trend < 0.0001), and biventricular ICDs (p trend = 0.02).” The authors concluded that “patients who have an ICD implanted at a high-volume hospital are less likely to have an adverse event associated with the procedure than patients who have an ICD implanted at a low-volume hospital.”
Al-Khatib S, Hellkamp M, Curtis J, et al. Non–evidence-based ICD implantations in the United States. JAMA. 2011;305(1):43-49.
The authors performed a retrospective cohort analysis to determine the number, characteristics, and in-hospital outcomes of patients who receive a non–evidence-based ICD and examine the distribution of these implants by site, physician specialty, and year of procedure. The study included 111,707 patients from the NCDR who received an ICD implantation between January 2006 and June 2009. The primary outcome was any in-hospital outcome, including death, any post-procedure complication, cardiac tamponade, pneumothorax, infection, hematoma, and length of hospital stay. Patients were classified as receiving a non–evidence-based ICD implant if they met at least one of following criteria: (1) had an MI within 40 days before ICD implantation; (2) had CABG surgery within 3 months before ICD implantation; (3) had NYHA class IV symptoms; or (4) had newly diagnosed heart failure at the time of ICD implantation. Patients who did not receive evidence-based ICD implantation were compared to those patients who did.
Study results demonstrated that 22.5% or the 111,707 study patients received non–evidence-based ICD implantation. “Patients who received a non–evidence-based ICD compared with those who received an evidence-based ICD had a significantly higher risk of in-hospital death (0.57% [95% CI 0.48%-0.66%] vs 0.18% [95% CI 0.15%-0.20%]; P <.001) and any post-procedure complication (3.23% [95% CI 3.01%-3.45%] vs 2.41% [95% CI 2.31%-2.51%]; P <.001).” There was substantial variation in non–evidence-based ICDs by site. The rate of non–evidence-based ICD implants was significantly lower for electrophysiologists than non-electrophysiologists. There was no clear decrease in the rate of non–evidence-based ICDs over time.
The authors concluded that “a substantial number of ICDs were implanted in patients who were similar to those who either were excluded from major clinical trials of primary prevention ICDs or shown not to benefit from ICD therapy in other trials. Such patients not only have more comorbidities than patients receiving an evidence-based device, but they are at a higher risk of in-hospital death and any post-procedure complication.” They also found no clear decrease in the overall number of non–evidence-based ICD implants over time. Thus the authors conclude that more effort is needed to improve physician adherence to evidence-based practice.
4. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
A MEDCAC meeting was not convened on this issue.
5. Evidence-Based Guidelines
American College of Cardiology, American Heart Association, and Heart Rhythm Society Guidelines
Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Hearth Rhythm Society (HRS) released the 2017 Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. An independent evidence review committee (ERC) was commissioned with the goal to determine which patients are most likely to benefit from a test, medication, device, or treatment strategy and to what degree. The writing committee considered the results of the ERC review, as well as other published data when developing the guideline recommendations. This guideline supersedes sections of the ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities regarding indications for ICDs, and updates SCD recommendations from the 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy.
The Class of Recommendation (COR) indicates the strength of the recommendation and estimates the magnitude of benefit versus risk.
- Class 1 (Strong): Is recommended. Should be performed/administered.
- Class IIa (Moderate): Is reasonable. Can be useful/effective/beneficial.
- Class IIb (Weak): May/might be reasonable. Usefulness/effectiveness is unknown/unclear/uncertain or not well established.
- Class III: No Benefit (Modearte): Is not recommended. Is not indicated/useful/effective/beneficial.
- Class III: Harm (Strong): Potentially harmful/Causes harm. Should not be performed/administered/other.
The Level of Evidence (LOE) rates the quality of the evidence based on the type, quantity, and consistency of the data from clinical trials and other sources.
- Level A
- High-quality evidence from more than 1 RCT
- Meta-analyses of high quality RCTs
- One or more RCTs corroborated by high-quality registry studies
- Level B-R
- Moderate-quality evidence from 1 or more RCTs
- Meta-analyses of moderate-quality RCTs
- Level B-NR
- Moderate-quality evidence from 1 or more well-designed, well-executed nonrandomized studies, observational studies, or registry studies
- Meta-analysis of such studies
- Level C-LD
- Randomized or nonrandomized observational or registry studies with limitations of design or execution
- Meta-analyses of such studies
- Physiological or mechanistic studies in human subjects
- Level C-EO
- Consensus of expert opinion based on clinical experience
The following recommendations were put forward
CLASS1
- In patients with ischemic heart disease, who either survive SCA due to VT/VF or experience hemodynamically unstable VT (LOE: B-R) (1-4) or stable VT (LOE: B-NR) (5) not due to reversible causes, an ICD is recommended if meaningful survival greater than 1 year is expected.
- In patients with ischemic heart disease and unexplained syncope who have inducible sustained monomorphic VT on electrophysiological study, an ICD is recommended if meaningful survival of greater than 1 year is expected (7) (LOE: B-NR).
- In patients with LVEF of 35% or less that is due to ischemic heart disease who are at least 40 days’ post-MI and at least 90 days postrevascularization, and with NYHA class II or III HF despite GDMT, an ICD is recommended if meaningful survival of greater than 1 year is expected (1, 2) (LOE: A).
- In patients with LVEF of 30% or less that is due to ischemic heart disease who are at least 40 days’ post-MI and at least 90 days postrevascularization, and with NYHA class I HF despite GDMT, an ICD is recommended if meaningful survival of greater than 1 year is expected (2, 3) (LOE: A).
- In patients with NSVT due to prior MI, LVEF of 40% or less and inducible sustained VT or VF at electrophysiological study, an ICD is recommended if meaningful survival of greater than 1 year is expected (5) (LOE: B-R).
- In patients with NICM who either survive SCA due to VT/VF or experience hemodynamically unstable VT (LOE: B-R) (1-4) or stable VT (LOE: B-NR) (5) not due to reversible causes, an ICD is recommended if meaningful survival greater than 1 year is expected.
- In patients with NICM, HF with NYHA class II–III symptoms and an LVEF of 35% or less, despite GDMT, an ICD is recommended if meaningful survival of greater than 1 year is expected (1-6) (LOE: A).
- In patients with HCM who have survived an SCA due to VT or VF, or have spontaneous sustained VT causing syncope or hemodynamic compromise, an ICD is recommended if meaningful survival greater than 1 year is expected (1, 6, 9, 10) (LOE: B-NR).
- In patients with cardiac sarcoidosis who have sustained VT or are survivors of SCA or have an LVEF of 35% or less, an ICD is recommended, if meaningful survival of greater than 1 year is expected (1-5) (LOE: B-NR).
- In patients with neuromuscular disorders, primary and secondary prevention ICDs are recommended for the same indications as for patients with NICM if meaningful survival of greater than 1 year is expected (1, 2) (LOE: B-NR).
- In patients with a cardiac channelopathy and SCA, an ICD is recommended if meaningful survival of greater than 1 year is expected (7-13) (LOE: B-NR).
- In high-risk patients with symptomatic long QT syndrome in whom a beta blocker is ineffective or not tolerated, intensification of therapy with additional medications (guided by consideration of the particular long QT syndrome type),
- left cardiac sympathetic denervation, and/or an ICD is recommended (2, 6-12) (LOE: B-NR).
- In patients with catecholaminergic polymorphic ventricular tachycardia and recurrent sustained VT or syncope, while receiving adequate or maximally tolerated beta blocker, treatment intensification with either combination medication therapy (e.g., beta blocker, flecainide), left cardiac sympathetic denervation, and/or an ICD is recommended (2-6) (LOE: B-NR).
- In patients with Brugada syndrome with spontaneous type 1 Brugada electrocardiographic pattern and cardiac arrest, sustained VA or a recent history of syncope presumed due to VA, an ICD is recommended if a meaningful survival of greater than 1 year is expected (4, 6) (LOE: B-NR).
- In patients with early repolarization pattern on ECG and cardiac arrest or sustained VA, an ICD is recommended (3, 4) (LOE: B-NR).
- In patients with short QT syndrome who have a cardiac arrest or sustained VA, an ICD is recommended if meaningful survival greater than 1 year is expected (3-5) (LOE: B-NR).
- In patients resuscitated from SCA due to idiopathic polymorphic VT or VF, an ICD is recommended if meaningful survival greater than 1 year is expected (9- 13) (LOE: B-NR).
- In patients with adult congenital heart disease and hemodynamically unstable VT, an ICD is recommended after evaluation and appropriate treatment for residual lesions/ventricular dysfunction if meaningful survival of greater than 1 year is expected (13-17) (LOE: B-NR).
- In patients with adult congenital heart disease with SCA due to VT or VF in the absence of reversible causes, an ICD is recommended if meaningful survival of greater than 1 year is expected (13-17) (LOE: B-NR).
- In patients who meet criteria for an ICD who have inadequate vascular access or are at high risk for infection, and in whom pacing for bradycardia or VT termination or as part of CRT is neither needed nor anticipated, a subcutaneous implantable cardioverter-defibrillator is recommended (1-5) (LOE: B-NR).
- Patients considering implantation of a new ICD or replacement of an existing ICD for a low battery should be informed of their individual risk of SCD and nonsudden death from HF or noncardiac conditions and the effectiveness, safety, and potential complications of the ICD in light of their health goals, preferences and values (1-5) (LOE: B-NR).
European Society of Cardiology Guidelines
Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793-867.
In ESC guidelines, “Class I” is defined as “evidence and/or general agreement that a given treatment or procedure is beneficial, useful, effective” and means that the intervention “is recommended” or “is indicated.” In turn, “Level of evidence A” is “data derived from multiple randomized clinical trials or meta-analyses” while level B is “data derived from a single randomized clinical trial or large non-randomized studies.” For definitions of all classes and levels of evidence, see the 2015 ESC guideline.
Secondary prevention of sudden cardiac death
For “secondary prevention of sudden cardiac death and ventricular tachycardia,” the ESC states that ICD implantation “is recommended” (Class 1, Level A) in patients “with documented VF or hemodynamically not tolerated VT in the absence of reversible causes or within 48 h after myocardial infarction who are receiving chronic optimal medical therapy and have a reasonable expectation of survival with a good functional status >1 year.”
ESC states that ICD implantation “should be considered” (Class IIa, Level C) in patients “with recurrent sustained VT (not within 48 h after myocardial infarction) who are receiving chronic optimal medical therapy, have a normal LVEF and have a reasonable expectation of survival with good functional status for >1 year.” [ESC 1618]
On “sustained VT,” ESC states: “implantation of an ICD in patients with sustained VT increases survival compared with anti-arrhythmic drug therapy. To date, no trial has been conducted comparing catheter ablation for sustained VT without ICD implantation and ICD placement only. In view of the scarcity of data and the rather high rate of recurrence following catheter ablation for sustained VT, ICD implantation should be considered in all patients with LV dysfunction (ejection fraction <45%) and sustained VT.”
Primary prevention of sudden cardiac death
For primary prevention of sudden cardiac death, ESC states that “ICD therapy is recommended to reduce SCD in patients with symptomatic HF (NYHA class II–III) and LVEF ≤35% after ≥3 months of optimal medical therapy who are expected to survive for at least 1 year with good functional status:
- Ischemic etiology (at least 6 weeks after myocardial infarction) – Class I, Level A
- Nonischemic etiology – Class I, Level B”
Note that these ESC Guidelines were updated in 2015 and thus do not consider the DANISH trial, reported in 2016.
For patients who are asymptomatic or have preserved left ventricular function, ESC states: “currently there are no RCTs demonstrating the value of an ICD in asymptomatic patients (NYHA class I) with systolic dysfunction (LVEF ≤35–40%) or in patients with HF and preserved LVEF >40–45%, so ICDs are not recommended for primary prevention in these patients.”
Special cases
For patients with New York Heart Association Class IV listed for heart transplantation, ESC states that ICD implantation “should be considered” (Class IIa, Level C) “for primary and secondary prevention of SCD in patients who are listed for heart transplant.”
ESC also supports consideration of ICD implantation for a number of familial or genetic diseases.
ESC states that subcutaneous defibrillators “should be considered” (Class IIa, Level C) “as an alternative to transvenous defibrillators in patients with an indication for an ICD when pacing therapy for bradycardia support, cardiac resynchronization or antitachycardia pacing is not needed.” ESC states that subcutaneous defibrillators “may be considered” (Class IIb, Level C) “as a useful alternative to the transvenous ICD system when venous access is difficult, after the removal of a transvenous ICD for infections or in young patients with a long-term need for ICD therapy.”
ESC states that wearable cardioverter defibrillators “may be considered” (Class IIb, Level C) for adult patients with poor LV systolic function who are at risk of sudden arrhythmic death for a limited period, but are not candidates for an implantable defibrillator (e.g. bridge to transplant, bridge to transvenous implant, peripartum cardiomyopathy, active myocarditis and arrhythmias in the early post-myocardial infarction phase). In discussing “gaps in evidence,” ESC further states that “wearable defibrillators may be an interesting therapeutic option in selected patients but require larger randomized trials before clear indications can be fully defined.”
Psychosocial management after ICD implantation
ESC states that: “assessment of psychological status and treatment of distress are recommended in patients with recurrent inappropriate shocks;” and “discussion of quality-of-life issues is recommended before ICD implantation and during disease progression in all patients.” (Both Class I, Level C.)
6. Professional Society Recommendations / Consensus Statements / Other Expert Opinion
Expert Consensus Statement
Kusumoto FM, Calkins H, Boehmer AE, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130(1):94-125.
The writing group included members who are considered leaders in their field and were selected from the Heart Rhythm Society (HRS), American College of Cardiology (ACC), American Heart Association (AHA), Heart Failure Society of America (HFSA), and the Society of Thoracic Surgeons (STS). The group evaluated data to provide clinicians with guidance on four situations where ICD therapy might be beneficial in selected populations of patients who are not represented in clinical trials. The four situations evaluated were: 1) use of an ICD in patients with an abnormal troponin that is not due to a MI, 2) use of an ICD within 40 days after a MI, 3) use of an ICD within the first 90 days after revascularization, and 4) use of an ICD in the first 9 months after initial diagnosis of nonischemic cardiomyopathy.
The writing group recommends implantation of an ICD in the following patient populations:
- Patients with abnormal cardiac biomarkers that are not thought to be due to an MI and who otherwise would be candidates for implantation on the basis of primary prevention or secondary prevention criteria.
- Patients who, within 40 days of an MI, require non-elective permanent pacing, who also would meet primary prevention criteria for implantation of an ICD, and recovery of left ventricular function is uncertain or not expected.
- Patients who, within 40 days of an MI, develop sustained (or hemodynamically significant) ventricular tachyarrhythmias > 48 hours after an MI and in the absence of ongoing ischemia.
- Patients within 40 days of an MI and who have an ICD that requires elective replacement due to battery depletion, after careful assessment of comorbidities and the current clinical situation.
- Patients within 90 days of revascularization who have previously qualified for the implantation of an ICD for secondary prevention of SCD (resuscitated from cardiac arrest due to ventricular tachyarrythmia) and have abnormal left ventricular function.
- Patients within 90 days of revascularization who have previously qualified for the implantation of an ICD for secondary prevention of SCD (resuscitated from cardiac arrest due to ventricular tachyarrythmia) that is unlikely related to myocardial ischemia/injury and have normal left ventricular function.
- Patients within 90 days of revascularization who require nonelective permanent pacing, who would also meet primary prevention criteria for implantation of an ICD, and in whom recovery of LV function is uncertain or not expected.
- Patients within 90 days of revascularization with structural heart disease and sustained (or hemodynamically significant) VT that was not clearly related to acute MI or ischemia.
- Patients within 90 days of revascularization with an ICD that requires replacement due to battery depletion, after careful assessment of comorbidities and the current clinical situation.
- Patients < 9 months from the initial diagnosis of nonischemic cardiomyopathy who require nonelective permanent pacing, who would meet primary prevention criteria for implantation of an ICD, and recovery of LV function is uncertain or not expected.
- Patients < 9 months from the initial diagnosis of nonischemic cardiomyopathy with sustained (or hemodynamically significant) ventricular tachyarrhythmia.
Appropriate Use Criteria
Russo AM, Stainback RF, Bailey SR et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 Appropriate use criteria for implantable cardioverter- defibrillators and cardiac resynchronization therapy. Heart Rhythm. 2013 Apr;10(4):e11-58.
The American College of Cardiology Foundation, the Heart Rhythm Society, and other specialty/ subspecialty societies conducted a review of common clinical scenarios where ICDs and CRT are considered. As stated by the writing committee, “The clinical scenarios covered in this document address secondary prevention, primary prevention, comorbidities, generator replacement at elective replacement indicator, dual-chamber ICD, and CRT.”
7. Public Comment
Public comments sometimes cite the published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination.
CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum. All comments that were submitted without personal health information may be viewed in their entirety by using the following link https://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=288.
Initial Comment Period: 5/30/2017 – 6/29/2017
During the initial 30-day public comment period, CMS received 36 comments. Of these 36 comments, one was omitted from publication on the CMS website due to excessive personal health information content, and one commenter posted twice. Most of the comments suggested changes to the language in the current NCD from 2005 for implantable defibrillators. Several of these comments asked for CMS to update the covered indications based on current professional guidelines, and numerous commenters supported ending the registry requirement for ICD implantation. A number of commenters also made reference to the DANISH study and asked that CMS not limit coverage for patients with non-ischemic cardiomyopathy based on the findings from one trial using subgroup analyses that were not sufficiently powered and including patients with substantially elevated NT-proBNP who may have been more likely to die of non-SCD causes.
The majority of comments were provided by physicians/cardiologists, electrophysiologists, and other healthcare professionals. There were three comments that represented five professional societies, including the American Heart Association (AHA), American Stroke Association (ASA), Heart Failure Society of America (HFSA), Heart Rhythm Society (HRS), and American College of Cardiology (ACC). Additional groups who offered comments were AdvaMed, Emory Healthcare, Path to Improved Risk Stratification, and Mercy Health. We also received three comments from manufacturers of ICDs, including Medtronic, Boston Scientific, and Abbott.
IX. CMS Analysis
Introduction: National coverage determinations are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1869(f)(1)(B) of the Act). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member (§1862(a)(1)(A) of the Act).
When making national coverage determinations, we evaluate the evidence related to our analytic questions based on the quality, strength and totality of evidence presented in the reviewed literature. As part of this evaluation, it is important to consider whether the evidence is relevant to the Medicare beneficiary population. In determining the generalizability of the results of the body of evidence to the Medicare population, we consider, at minimum, the age, race and gender of the study participants.
Evidence Review Summary: Existing Medicare coverage policies and professional medical society guidelines alike are based on evidence from randomized controlled trials that support the use of ICDs in patients with heart failure and either ischemic or non-ischemic dilated cardiomyopathy. However, we note that many of these trials were conducted 15-20 years ago and since that time medical therapy has evolved substantially. Since our last decision, several meta-analyses, systematic evidence reviews and technology assessments have been published and provide evidence that ICDs are reasonable and necessary for certain patients aligning with our prior criteria. In general, meta-analysis when designed appropriately and performed rigorously can provide high strength evidence.
Questions: For this reconsideration, CMS focused on the following questions:
- Is there evidence to conclude that ICDs decrease mortality for patients with ischemic dilated cardiomyopathy (IDCM) and reduced LVEF?
- Is there evidence to conclude that ICDs decrease mortality for patients with non-ischemic cardiomyopathy (NIDCM) and reduced LVEF?
Is there evidence to conclude that ICDs decrease mortality for patients with ischemic dilated cardiomyopathy (IDCM) and reduced LVEF?
Yes. Uhlig and colleagues (2013) analyzed 14 trials including IRIS and concluded that “there is a high strength of evidence that ICD therapy for primary prevention of SCD, versus no ICD therapy, shows benefit with regard to all-cause mortality and SCD in patients with reduced left ventricular ejection fraction and ischemic or non-ischemic cardiomyopathy beyond the immediate post-MI or coronary revascularization periods.” Colquitt and colleagues (2014) analyzed 13 trials including IRIS and found that “ICDs reduced all-cause mortality in people at increased risk of SCD, defined in trials as those with previous ventricular arrhythmias/cardiac arrest, myocardial infarction (MI) > 3 weeks previously, non-ischemic cardiomyopathy (depending on data included) or ischemic/non-ischemic heart failure (HF) and left ventricular ejection fraction ≤ 35%.”
Specifically for patients with heart failure and non-ischemic cardiomyopathy, Al-Khatib and colleagues (2017) analyzed four trials including DANISH and showed that “use of an ICD significantly reduced all-cause mortality (HR 0.75; CI 0.61-0.93; P=.008, P=0.87 for heterogeneity).” Golwala and colleagues (2017) analyzed six trials including DANISH and showed that “use of an ICD significantly reduced all-cause mortality by 23% (HR 0.77; CI 0.64–0.91).”
Is there evidence to conclude that ICDs decrease mortality for patients with non-ischemic cardiomyopathy (NIDCM) and reduced LVEF?
Yes. Overall, based on the preponderance of evidence including past randomized controlled trials, new trials, meta-analyses, systematic evidence reviews and technology assessments, we propose that the evidence is sufficient to conclude that ICDs decrease mortality for symptomatic patients with severe ischemic and severe non-ischemic dilated cardiomyopathy and reduced LVEF.
We believe the proposed decision reflects the currently available evidence, including professional society guidelines. Further, by adding patient shared decision making we are empowering patients to be part of their health treatment decisions. In addition, by removing the data collection we are reducing the burden on providers.
The general criteria from the 2005 NCD which applies to all categories of patients who are candidates for an ICD are mostly unchanged or represent minor modifications based on recent published consensus guidelines by professional medical societies, evidence reviewed and/or public comments. We go into detail below regarding covered indications.
Patients with a Prior Personal History of Cardiac Arrest or Sustained Ventricular Tachyarrhythmia: A literature search did not find any new trials since the 2005 NCD relevant to the clinical utility of ICDs for symptomatic patients with a prior personal history of sudden cardiac arrest or sustained ventricular tachyarrhythmias. Therefore, we propose no changes to this section of the decision.
Patients with a prior MI and a measured LVEF ≤ 0.30: There were several systematic reviews and meta-analyses assessing these patients with prior MI and a measured LVEF ≤ 0.30. These analyses confirmed the benefit of ICDs in these patients. Therefore, we are proposing no changes in coverage.
Patients Who Have Severe Ischemic and/or Non-Ischemic Dilated Cardiomyopathy but No Prior Personal History: For symptomatic patients who have ischemic and non-ischemic dilated cardiomyopathy but no prior personal history of sudden cardiac arrest is minimally changed from our existing coverage policy. As explained below, we propose to add that these patients must have at least 3 months of optimal medical therapy to reflect current advances in cardiac care.
For Medicare patients with ischemic cardiomyopathy, the baseline standard of care of MADIT II (2002) and SCD-HeFT (2005) may no longer reflect the contemporary standard of care (Bardy et al, 2005; Moss et al., 2002). However, other than IRIS (2009), which demonstrated that ICDs do not improve survivability if implanted within 31 days after myocardial infarction, confirming the results of DINAMIT (2004) and supporting our prior coverage criterion, no other major trials have been completed assessing the clinical utility of ICDs versus optimal medical therapy in patients with ischemic cardiomyopathy, since the 2005 reconsideration (Hohnloser et al, 2004; Steinbeck et al., 2009).
Results from the DANISH study called into question the utility of ICDs for certain patients with heart failure and non-ischemic cardiomyopathy. While we agree that DANISH contributed to the evidence base for the treatment of heart failure patients at risk for SCD, there are several factors to consider: 1) the study population may be different with the inclusion of NT-proBNP and 58% CRT composition of DANISH in the ICD and control groups; 2) DANISH was not powered for subgroup analyses on age and CRT status; and 3) the absence of a parallel registry made it difficult to determine whether eligible patients who enrolled were similar to those who were not.
Two aspects of the baseline optimal medical therapy in the DANISH trial stand out. First, it is the only trial that includes CRT as a component of baseline optimal medical therapy. While an important consideration, cardiac resynchronization therapy is distinct and generally beyond the scope of this decision. Second, it reflects the marked advancement of pharmacological therapy that has occurred since the earlier ICD trials. As with other medical conditions, better medical treatments and care develops over time with evidence and experience. The determination of effect of these changes on trial results compared to prior trials is a topic of discussion which will not be answerable in this decision. Importantly, with additional treatment options, a detailed discussion of the potential benefits and harms with each patient would be a key step to choosing the best treatment for any particular individual.
The requirement of being on optimal medical therapy for at least three months is in the inclusion criteria of multiple trials, including the 2016 DANISH trial, and we believe it is reasonable for ischemic and non-ischemic heart failure patients alike. Therefore, we are proposing that patients must be on optimal medical therapy at least three months before implantation of an ICD because of the advances in optimal therapy for cardiac care.
We found no evidence to support making any other changes to existing CMS coverage policy on the use of ICDs for patients with ischemic and/or non-ischemic cardiomyopathy, or to make changes to the LVEF requirements from the 2005 reconsideration.
Other Clinical Circumstances: This proposed decision provides the same coverage of ICDs for patients with no prior history and familial or genetic disorders. As we did in the prior NCD, we considered the rationale, evidence, benefits and harms, professional society recommendations and consensus guidelines. Based on the evidence we propose no changes.
The requirement of patients having NYHA Class IV heart failure for CRT was removed in this proposed decision since we believe CRT is a separate therapy and should be evaluated in a distinct determination. Because there is no national coverage criteria for CRT, we are proposing to remove this section of the NCD.
General Patient Criteria: Based on the evidence reviewed and the 2017 society guidelines, we made no changes for eligible clinically stable patients requirement. For eligible clinically stable patients, we propose no changes from the 2005 ICD reconsideration. We continue to require that these patients must not have significant irreversible brain damage or any disease other than cardiac disease associated with a likelihood of survival of less than one year. We added cardiac MRI to the list of diagnostic imaging studies that can evaluate left ventricular ejection fraction, based on new evidence in part cited in recent professional medical society guidelines and public comments (Al-Khatib et al., 2017; Russo et al., 2013).
Exceptions to Waiting Periods: We are proposing to make certain exceptions to the waiting period requirements for symptomatic patients who have ischemic and non-ischemic dilated cardiomyopathy but no prior personal history of sudden cardiac arrest (see sections B2 and B3 of the proposed decision in Section I), acknowledging that the wording in the 2005 decision could inadvertently require a second procedure when only one procedure is needed. In patients with an existing ICD who then suffer an MI or undergo a coronary revascularization procedure, we would now allow replacement of the device, if clinically indicated, waiving the previously mentioned waiting periods. This exception also applies to patients without an existing ICD who meet requirements for both a cardiac pacemaker and a defibrillator; thus, if the pacemaker is needed for pacing needs, the defibrillator may be implanted in the same procedure, and a patient would no longer be required to undergo a second procedure for the defibrillator after the expiration of the waiting periods. This is standard medical practice and recommended in the HRS/ACC/AHA expert consensus statement (Kusumoto et al. 2014). We note that specific coverage criteria for pacemakers goes beyond the scope of this NCD, which focuses on the clinical utility of ICDs.
We are also proposing an exception to the required waiting periods for patients with an existing ICD (see section B2 of the proposed decision in Section I) that requires replacement due to the end of battery life, elective replacement indicator (ERI), or device malfunction, with documentation that the device is at ERI level, or that there is a device/lead malfunction. This is practical medical practice and recommended in the HRS/ACC/AHA expert consensus statement (Kusumoto et al. 2014).
Patient Shared Decision Making: Since there are some outstanding questions regarding the appropriate populations benefitting from ICDs, we are proposing to include a requirement for a patient shared decision making (SDM) interaction into our decision for certain patient populations. We do not believe an SDM interaction would be beneficial for patients with a prior personal history of cardiac arrest or sustained ventricular tachyarrhythmia given the lack of alternative treatment options, nor do we believe SDM is necessary for patients with an existing ICD who qualify for a replacement ICD since a previous implantation had already occurred. SDM is especially important in treatments where there are complex considerations on benefits, harms, indications and existing effective treatments. Barry and Edgman-Levitan (2012) noted: “[t]he process by which the optimal decision may be reached for a patient at a fateful health crossroads is called shared decision making and involves, at minimum, a clinician and the patient, although other members of the health care team or friends and family members may be invited to participate. In shared decision making, both parties share information: the clinician offers options and describes their risks and benefits, and the patient expresses his or her preferences and values. Each participant is thus armed with a better understanding of the relevant factors and shares responsibility in the decision about how to proceed.” Ideally SDM integrates the use of evidence-based decision tools including treatment pictograms to characterize benefits and harms.
The importance of individual patient values and preferences in decision making applies to the use of ICDs. As endorsed in the joint 2017 guidelines by AHA/ACC/HRS, “In patients with VA [Ventricular Arrhythmia] or at increased risk for SCD, clinicians should adopt a shared decision-making approach in which treatment decisions are based not only on the best available evidence but also on the patients’ health goals, preferences, and values”. SDM must occur prior to seeking ICD implantation, and be documented in the medical records.
An example of an existing SDM tool for ICDs is the joint effort between The Colorado Program for Patient Centered Decisions, with funding from the National Institutes on Aging (K23AG040696) and the Patient-Centered Outcomes Research Institute (PI000116-01).to develop an evidence-based decision aid tool for patients with heart failure considering an ICD who are at risk for sudden cardiac death (primary prevention). https://patientdecisionaid.org/wp-content/uploads/2017/01/ICD-Infographic-5.23.16.pdf. The decision aid tools and website were developed based on research study findings and interviews with patients.
In addition to the tool, they developed a website which leads patients step-by-step through some information on ICDs designed to increase patients’ knowledge of their medical condition, the risks and benefits of available treatments and to empower patients to become more involved in the decision-making process. https://patientdecisionaid.org/icd/.
Registry Requirement: In 2005, CMS had some questions about the evidence (see Appendix C for the current 20.4 NCD). Based on our concerns at the time, we required additional data to be collected via a registry (see https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=110&ncdver=3&NCAId=288&bc). We assessed the extent to which the published literature has addressed the following ten “initial hypotheses” for the registry data collection requirement in the 2005 NCD. Based on our analysis, the eight peer-reviewed publications were directly related to the 10 initial hypotheses have been answered. CMS believes that additional data collection is no longer needed. We have listed the 10 hypotheses below for the ease of the reader. In addition, we have summarized the publications and have explained why they answered the hypostheses.
- The clinical characteristics of the patients receiving ICDs are similar to those of patients involved in the primary prevention randomized clinical trials.
- The indications for ICD implantation in patients are similar to those in the primary prevention randomized clinical trials.
- The in-hospital procedure related complications for patients are similar to those in the primary prevention randomized clinical trials.
- Certified providers competent in ICD implantation are implanting ICD devices in patients.
- Patients who receive an ICD represent patients for which current clinical guidelines and the evidence base recommend implantation.
- The clinical characteristics and indications for ICD implantation do not differ significantly among facilities.
- The clinical characteristics and indications for ICD implantation do not differ significantly among providers.
- The in-hospital procedure related complications for ICD implantation do not differ significantly among facilities.
- The in-hospital procedure related complications for ICD implantation do not differ significantly among providers.
- The in-hospital procedure related complications for ICD implantation do not differ significantly among device manufacturer, types, and/or programming.
In sum, we believe the research questions for each of the studies specifically listed in the Evidence Section addresses one or more of the ten CMS “initial hypotheses;” and collectively, these eight studies are related to all ten hypotheses. Again, we are aware that there are numerous other published studies that may be related to one or more of these hypotheses, or aspects of them. These real world studies have provided support for implementation of this technology outside the controlled trial environment. The initial data collection requirement through the NCDR has served to generate and improve the evidence base for the use of ICDs in certain Medicare beneficiaries. We acknowledge the substantial contribution of the NCDR, the input of a number of professional societies and contribution of manufacturers in this positive collaborative effort over the past decade. We believe it has served its purpose and are proposing to end the registry data collection requirement. However, we encourage the continuation and improvement of a voluntary registry for purposes of quality improvement, safety, and appropriate use verification.
Considerations for Further Research: Based on our analysis, we recognize that further research could be done on risk stratification and specific subpopulations. We also recognize the separate but related CRT technology. In discussion of research gaps in a 2015 AHRQ-funded Technology Assessment, Rickard et al conclude: “The effectiveness of CRT-D versus CRT-P in patients with an LVEF ≤35% has not been adequately addressed.” In the 2014 NICE Health Technology Assessment, Colquitt et al conclude: “A RCT comparing CRT-D [CRT with a defibrillator] and CRT-P [a CRT pacemaker alone] in people with heart failure as a result of left ventricular systolic dysfunction and cardiac dyssynchrony is required, for both those with and those without an ICD indication.
While the 2016 DANISH trial has made a new contribution to this evidence base, Kober et al, concluded it is unclear whether patients who are eligible for CRT should routinely receive an ICD, and a randomized study (CRT-D to CRT-P) would be clinically important. Accordingly, we believe randomized controlled trials comparing CRT-D to CRT-P on the backdrop of contemporary, optimal pharmacological therapy in specific patient populations with dilated cardiomyopathy would be informative. Such trials would provide evidence and supporting documentation for a separate consideration of CRT.
We appreciate the importance of further research on a myriad of potential risk stratification models that combine clinical and demographic data with data from various studies (such as laboratory tests, interventional electrophysiological (EP) studies, cardiac magnetic resonance imaging (MRI), to name just a few), whether alone or in some combination or sequence, and for particular populations. As noted by Uhlig and colleagues in their 2013 technology assessment: “Prevention is the primary strategy to lower death from SCD. However, SCD is a particular management challenge because the majority of cases occur in individuals without a prior diagnosis of cardiac disease or other clear risk factors for SCD. The most common underlying cardiovascular diagnosis among people with SCD is coronary artery disease (CAD). Yet, in about half of the cases of SCD, SCD itself is the initial manifestation of CAD. The clinical strategy to prevent death from SCD involves identification of risk factors for ventricular tachyarrhythmias and SCD, to target individuals for medical and interventional treatments.”
While we encourage such research on risk stratification to continue, we acknowledge that other agencies are better equipped and have clearer authority to take the lead in vetting and supporting such a large and varied research portfolio, some of which is in earlier discovery and testing phases. However, it is important to note that all other indications for ICDs not currently covered in accordance with this decision may continue to be covered under Category B IDE trials (42 CFR 405.201). The end result of such research could improve health outcomes of Medicare beneficiaries.
Health Disparities
Current research has not addressed the disparities of ICD implantation in African Americans when compared to Caucasians. Despite being at higher risk for SCD, Hernandez et al. (2007) found that African Americans who were potentially eligible for an ICD were 30% less likely to receive an ICD compared to Caucasians, independent of other characteristics. Hernandez et al. (2007) also observed this disparity in women who were potentially eligible for an ICD, finding that they were 40% less likely than men to undergo ICD therapy. African Americans and women are both traditionally underrepresented in research and these findings illustrate the need for future research to address these disparities.
Summary
This NCA has focused on the use of ICDs in symptomatic patients who have severe ischemic and non-ischemic dilated cardiomyopathy with no prior history of cardiac arrest or sustained ventricular tachyarrhythmias. As we have discussed, we propose to not substantively change the current coverage policy for ICDs for symptomatic patients with a prior personal history of cardiac arrest. We propose exceptions to the time requirement for patients who are pacemaker dependent and otherwise qualify for an ICD implant and for beneficiaries with an existing ICD which requires replacement, eliminating the possibility of unnecessary invasive procedures and allows for timely replacement of a potentially lifesaving system which requires replacement.
Additionally, we are proposing to include a shared decision making encounter to engage patients, to discuss their treatment options and the potential benefits and harms associated with ICDs, and to incorporate their views and beliefs in choosing the most appropriate treatment. Based on the evidence, we propose to reduce provider burden and documentation through discontinuation of the registry data collection requirement and the need to document ischemic cardiomyopathy etiology. While we agree that some research questions remain, the data and information gathered from the NCDR has substantially contributed to the evidence base and supports our proposal to end the registry data collection requirements from the 2005 NCD. We acknowledge that additional research questions remain that cannot be addressed by registries and we provide details about the study designs that could potentially address these questions. It is our understanding that such studies are currently being planned.
IX. Conclusion
-
The Centers for Medicare & Medicaid Services (CMS) proposes that the evidence is sufficient to conclude that the use of implantable cardioverter defibrillators (ICDs, also referred to as defibrillators) is reasonable and necessary for the treatment of illness or injury or to improve the functioning of a malformed body member under section 1862(a)(1)(A) of the Social Security Act.
CMS is proposing relatively minimal changes to the ICD NCD from the 2005 reconsideration. We summarized the changes below and fully explain the changes in the Analysis section of the NCD decision memo.
- Patient Criteria
- Adding cardiac MRI to the list of diagnostic imaging studies that can evaluate left ventricular ejection fraction (LVEF).
- Requiring patients who have severe ischemic and/or non-ischemic dilated cardiomyopathy but no prior personal history to have been on optimal medical therapy for at least 3 months;
- Requiring a patient shared decision making (SDM) interaction prior to ICD implantation for certain patients.
- Additional Patient Criteria
- Removing the Class IV heart failure requirement for CRT.
- Exceptions to Waiting Periods
- Adding an exception for patients meeting CMS coverage requirements for cardiac pacemakers, and who meet the criteria for an ICD;
- Adding an exception for patients with an existing ICD and qualifying replacement.
- Registry Requirement
- Ending the data collection requirement.
We are proposing changes to the 20.4 NCD that reflect the 2005 reconsideration as described below:
- Covered Indications
- Patients with a prior personal history of cardiac arrest or sustained ventricular tachyarrhythmia:
- A documented episode of cardiac arrest due to ventricular dysrhythmia (ventricular fibrillation [VF] or ventricular tachycardia [VT]), not due to a transient or reversible cause; or
- A documented episode of sustained VT not due to a transient or reversible cause, either spontaneous or induced by an electrophysiology (EP) study, in symptomatic patients, not associated with an Acute Myocardial Infarction (AMI), ≥4 days after revascularization if a revascularization procedure is performed, and with no evidence of ongoing ischemia.
- Patients with a prior MI and a measured LVEF ≤ 0.30. Patients must not have:
- New York Heart Association (NYHC) classification IV;
- Had a coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) with angioplasty and/or stenting, within the past 3 months; or
- Had a MI within the past 40 days; or
- Clinical symptoms and findings that would make them a candidate for coronary revascularization.
For these patients, a formal shared decision making encounter must occur between the patient and an independent physician (as defined in Section 1861(r)(1)) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5)) using an evidence-based decision tool on ICDs prior to initial ICD implantation.
- Patients who have severe ischemic and/or non-ischemic dilated cardiomyopathy but no prior personal history, NYHA Class II or III heart failure, left ventricular ejection fraction (LVEF) ≤ 35%, been on optimal medical therapy for at least 3 months. Additionally, patients who have severe ischemic and/or non-ischemic dilated cardiomyopathy but no prior personal history must not have:
- Had a coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) with angioplasty and/or stenting, within the past 3 months; or
- Had a MI within the past 40 days; or
- Clinical symptoms and findings that would make them a candidate for coronary revascularization.
For these patients, a formal shared decision making encounter must occur between the patient and an independent physician (as defined in Section 1861(r)(1)) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5)) using an evidence-based decision tool on ICDs prior to initial ICD implantation.
- Patients with documented familial, or genetic disorders with a high risk of life-threatening tachyarrhytmias (sustained VT or VF), to include, but not limited to, long QT syndrome or hypertrophic cardiomyopathy.
For these patients, a formal shared decision making encounter must occur between the patient and an independent physician (as defined in Section 1861(r)(1)) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5)) using an evidence-based decision tool on ICDs prior to initial ICD implantation.
- Patients with an existing ICD may receive an ICD replacement if it is required due to the end of battery life, elective replacement indicator (ERI), or device/lead malfunction.
For each of these groups listed above, the following additional criteria must also be met:
- Patients must be clinically stable (e.g., not in shock, from any etiology);
- LVEF must be measured by echocardiography, radionuclide (nuclear medicine) imaging, cardiac magnetic resonance imaging (MRI), or catheter angiography;
- Patients must not have:
- Significant, irreversible brain damage; or
- Any disease, other than cardiac disease (e.g., cancer, renal failure, liver failure) associated with a likelihood of survival less than 1 year; or
- Uncontrolled supraventricular tachycardia such as from atrial fibrillation.
- Exceptions to waiting periods specified in sections B2 and B3:
Cardiac Pacemakers: Patients who meet all CMS coverage requirements for cardiac pacemakers, and who meet the criteria in this national coverage determination for an ICD, may receive the combined devices in one procedure, at the time the pacemaker is clinically indicated;
Replacement of ICDs: Patients with an existing ICD may receive a ICD replacement if it is required due to the end of battery life, elective replacement indicator (ERI), or device malfunction, with documentation that the device is at ERI level, or that there is a device/lead malfunction.
All other indications for ICDs not currently covered in accordance with this decision may be covered under Category B IDE trials (42 CFR 405.201).
See Appendix B for the proposed manual language.
CMS is seeking comments on our proposed decision. We will respond to public comments in a final decision memorandum, as required by §1862(l)(3) of the Social Security Act (the Act).
APPENDIX A
General Methodological Principles of Study Design
(Section VI of the Decision Memorandum)
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to that group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is to the extent that differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well-designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of that have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
Randomized controlled trials
Non-randomized controlled trials
Prospective cohort studies
Retrospective case control studies
Cross-sectional studies
Surveillance studies (e.g., using registries or surveys)
Consecutive case series
Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in that confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to
our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but
would suffer from limited generalizability.
The extent to that the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of
interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
APPENDIX B
Medicare National Coverage Determinations Manual
Draft
We are seeking public comments on the proposed language that we would include in the Medicare National Coverage Determinations Manual. This proposed language does not reflect public comments that will be received on the proposed decision memorandum, and which may be revised in response to those comments.
Table of Contents
(Rev.)
A. General
An implantable cardioverter defibrillator is an electronic device designed to diagnose and treat life-threatening ventricular tachyarrhythmias. The device consists of a pulse generator and electrodes for sensing and defibrillating. This therapy has been shown in trials to improve survival and reduce sudden cardiac death in patients with certain clinical characteristics.
B. Nationally Covered Indications
Effective for services performed on or after [Month/XX] [Day/XX], [20XX] CMS proposes that the evidence is sufficient to conclude that the use of implantable cardioverter defibrillators (ICDs, also referred to as defibrillators) is reasonable and necessary, and proposes changes to the 20.4 NCD that reflect the 2005 reconsideration as described below:
Covered Indications
- Patients with a prior personal history of cardiac arrest or sustained ventricular tachyarrhythmia:
- A documented episode of cardiac arrest due to ventricular dysrhythmia (ventricular fibrillation [VF] or ventricular tachycardia [VT]), not due to a transient or reversible cause; or
- A documented episode of sustained VT not due to a transient or reversible cause, either spontaneous or induced by an electrophysiology (EP) study, in symptomatic patients, not associated with an Acute Myocardial Infarction (AMI), ≥4 days after revascularization if a revascularization procedure is performed, and with no evidence of ongoing ischemia.
- Patients with a prior MI and a measured LVEF ≤ 0.30. Patients must not have:
- New York Heart Association (NYHC) classification IV;
- Had a coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) with angioplasty and/or stenting, within the past 3 months; or
- Had a MI within the past 40 days; or
- Clinical symptoms and findings that would make them a candidate for coronary revascularization.
For these patients, a formal shared decision making encounter must occur between the patient and an independent physician (as defined in Section 1861(r)(1)) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5)) using an evidence-based decision tool on ICDs prior to initial ICD implantation.
- Patients who have severe ischemic and/or non-ischemic dilated cardiomyopathy but no prior personal history, NYHA Class II or III heart failure, left ventricular ejection fraction (LVEF) ≤ 35%, been on optimal medical therapy for at least 3 months. Additionally, patients who have severe ischemic and/or non-ischemic dilated cardiomyopathy but no prior personal history must not have:
- Had a coronary artery bypass graft (CABG), or percutaneous coronary intervention (PCI) with angioplasty and/or stenting, within the past 3 months; or
- Had a MI within the past 40 days; or
- Clinical symptoms and findings that would make them a candidate for coronary revascularization.
For these patients, a formal shared decision making encounter must occur between the patient and an independent physician (as defined in Section 1861(r)(1)) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5)) using an evidence-based decision tool on ICDs prior to initial ICD implantation.
- Patients with documented familial, or genetic disorders with a high risk of life-threatening tachyarrhytmias (sustained VT or VF), to include, but not limited to, long QT syndrome or hypertrophic cardiomyopathy.
For these patients, a formal shared decision making encounter must occur between the patient and an independent physician (as defined in Section 1861(r)(1)) or qualified non-physician practitioner (meaning a physician assistant, nurse practitioner, or clinical nurse specialist as defined in §1861(aa)(5)) using an evidence-based decision tool on ICDs prior to initial ICD implantation.
- Patients with an existing ICD may receive an ICD replacement if it is required due to the end of battery life, elective replacement indicator (ERI), or device/lead malfunction.
For each of these groups listed above, the following additional criteria must also be met:
- Patients must be clinically stable (e.g., not in shock, from any etiology);
- LVEF must be measured by echocardiography, radionuclide (nuclear medicine) imaging, cardiac magnetic resonance imaging (MRI), or catheter angiography;
- Patients must not have:
- Significant, irreversible brain damage; or
- Any disease, other than cardiac disease (e.g., cancer, renal failure, liver failure) associated with a likelihood of survival less than 1 year; or
- Uncontrolled supraventricular tachycardia such as from atrial fibrillation.
Cardiac Pacemakers: Patients who meet all CMS coverage requirements for cardiac pacemakers, and who meet the criteria in this national coverage determination for an ICD, may receive the combined devices in one procedure, at the time the pacemaker is clinically indicated;
Replacement of ICDs: Patients with an existing ICD may receive a ICD replacement if it is required due to the end of battery life, elective replacement indicator (ERI), or device malfunction, with documentation that the device is at ERI level, or that there is a device/lead malfunction.
All other indications for ICDs not currently covered in accordance with this decision may be covered under Category B IDE trials (42 CFR 405.201).


