To: Administrative File: CAG-00430N
From: Louis Jacques, MD
Director, Coverage and Analysis Group
Tamara Syrek Jensen, JD
Deputy Director, Coverage and Analysis Group
Jyme Schafer, MD, MPH
Director, Division of Medical and Surgical Services
Sarah Fulton, MHS
Lead Analyst, Division of Medical and Surgical Services
Lawrence Schott, MD, MS
Lead Medical Officer, Division of Medical and Surgical Services
Subject: Proposed Coverage Decision Memorandum for Transcatheter Aortic Valve Replacement (TAVR)
Date: February 2, 2012
I. Proposed Decision
The Centers for Medicare & Medicaid Services (CMS) proposes that coverage for TAVR be approved under Coverage with Evidence Development (CED) only for the following conditions and as specified below:
- TAVR is covered for the treatment of severe symptomatic aortic valve stenosis only, when all of the following conditions 1-5 are met.
- The procedure is furnished for an FDA approved indication, with a complete valve and implantation system that has received FDA premarket approval (PMA) for this indication.
- Two cardiac surgeons have, according to the pivotal PMA trial’s protocol, evaluated the patient’s suitability for open valve replacement surgery.
- The procedure is furnished in a facility that meets the following institutional requirements:
- For centers without previous PMA clinical trial TAVR experience
- Surgical program requirements:
- ≥ 50 total aortic valve replacement (AVR) procedures/year, including ≥ 10 patients with STS (Society of Thoracic Surgeons) Score ≥ 6;
- ≥ 2 institutionally based cardiac surgeons.
- Interventional program requirements:
- ≥ 400 caths/150 PCI’s (percutaneous interventions) per year;
- ≥ 15 left-sided structural (EVAR [endovascular aneurysm repair], TEVAR [thoracic endovascular aortic repair], etc.) interventions per year.
- For centers with previous PMA clinical trial TAVR experience
- Participation in ongoing TAVR programs, either randomized controlled trials (RCTs) or post-approval study (PAS);
- Experience with ≥ 30 TAVR procedures and ≥ 20/year;
- TAVR program requirements:
- ≥ 20 procedures/year OR ≥ 40 procedures/2 years;
- 30 day all-cause mortality ≤ 15%;
- 30 day neurologic events ≤ 15%;
- ≥ 90% institutional follow-up of patients;
- ≥ 60% one year survival for non-operable patients.
- For all centers, with or without previous PMA clinical trial TAVR experience:
- Participation in a prospective national TAVR study for ongoing enrollment and follow up of all TAVR patients;
- Commitment to Heart Team concept.
- The procedure is performed by physicians with the following qualifications and experience:
- Surgeon requirements:
- Board Certified/Eligible in Cardiovascular Surgery;
- Professional experience with:
- ≥ 100 AVR/career including 10 high risk patients; OR
- ≥ 25 AVR/year or 50 AVR in 2 years; AND
- ≥ 20 in the last year prior to TAVR.
- Interventionalist requirements:
- Operators must be Board Certified/Eligible in Interventional Cardiology
- Professional experience with 50 structural heart disease procedures
- The patient is enrolled in, and the treating physician team is participating in a prospective national registry that consecutively enrolls TAVR patients and tracks at least the following outcomes at the patient data level for a period of at least five years from the time of the TAVR procedure.
- Major stroke;
- All cause mortality;
- Minor stroke/TIA;
- Major vascular events;
- Acute kidney injury;
- Repeat aortic valve procedures;/li>
- Quality of Life measures.
The registry must be designed to permit identification and analysis of patient, practitioner and facility level factors that predict patient risk for these outcomes. The patient must have, after being informed of the reported risks of TAVR and reasonable alternative management strategies, given informed consent.
- Except as specified under A. above or C. below, CMS proposes coverage for all unlabeled uses of TAVR only when all of the following conditions are met:
- TAVR is covered in clinical studies that fulfill criteria a-m below and have characteristics i-ii.
- Superiority (not non-inferiority) TAVR study design; and
- Where TAVR is performed by a multi-disciplinary heart team that includes cardiologist(s) and cardiac surgeon(s) jointly participating in intra-operative technical aspects of TAVR.
The clinical study must adhere to the following standards of scientific integrity and relevance to the Medicare population:
- The principal purpose of the research study is to test whether a particular intervention potentially improves the participants’ health outcomes.
- The research study is well supported by available scientific and medical information or it is intended to clarify or establish the health outcomes of interventions already in common clinical use.
- The research study does not unjustifiably duplicate existing studies.
- The research study design is appropriate to answer the research question being asked in the study.
- The research study is sponsored by an organization or individual capable of executing the proposed study successfully.
- The research study is in compliance with all applicable Federal regulations concerning the protection of human subjects found in the Code of Federal Regulations (CFR) at 45 CFR Part 46. If a study is regulated by the Food and Drug Administration (FDA), it also must be in compliance with 21 CFR Parts 50 and 56. In particular, the informed consent includes a straightforward explanation of the reported increased risks of stroke and vascular complications that have been published for TAVR.
- All aspects of the research study are conducted according to appropriate standards of scientific integrity (see http://www.icmje.org).
- The research study has a written protocol that clearly addresses, or incorporates by reference, the standards listed as Medicare coverage requirements.
-
The clinical research study is not designed to exclusively test toxicity or disease pathophysiology in healthy individuals. Trials of all medical technologies measuring therapeutic outcomes as one of the objectives meet this standard only if the disease or condition being studied is life threatening as defined in 21 CFR § 312.81(a) and the patient has no other viable treatment options.
- The clinical research study is registered on the www.ClinicalTrials.gov website by the principal sponsor/investigator prior to the enrollment of the first study subject.
- The research study protocol specifies the method and timing of public release of all prespecified outcomes to be measured including release of outcomes if outcomes are negative or study is terminated early. The results must be made public within 24 months of the end of data collection. If a report is planned to be published in a peer reviewed journal, then that initial release may be an abstract that meets the requirements of the International Committee of Medical Journal Editors (http://www.icmje.org). However a full report of the outcomes must be made public no later than three (3) years after the end of data collection.
- The research study protocol must explicitly discuss subpopulations affected by the treatment under investigation, particularly traditionally underrepresented groups in clinical studies, how the inclusion and exclusion criteria affect enrollment of these populations, and a plan for the retention and reporting of said populations on the trial. If the inclusion and exclusion criteria are expected to have a negative effect on the recruitment or retention of underrepresented populations, the protocol must discuss why these criteria are necessary.
- The research study protocol explicitly discusses how the results are or are not expected to be generalizable to the Medicare population to infer whether Medicare patients may benefit from the intervention. Separate discussions in the protocol may be necessary for populations eligible for Medicare due to age, disability or Medicaid eligibility.
Consistent with section 1142 of the Act, the Agency for Healthcare Research and Quality (AHRQ) supports clinical research studies that CMS determines meet the above-listed standards and address the above-listed research questions.
- We propose national non-coverage of TAVR for all indications other than those noted above, and further specify non-coverage of TAVR in patients with:
- Mixed aortic valve disease (aortic stenosis and aortic regurgitation with predominant aortic regurgitation > 3+);
- Isolated aortic regurgitation;
- Untreated clinically significant coronary artery disease requiring revascularization;
- Hypertrophic cardiomyopathy with or without obstruction;
- Echocardiographic evidence of intracardiac mass, thrombus or vegetation;
- Significant aortic disease, including abdominal aortic or thoracic aneurysm defined as maximal luminal diameter ≥ 5 cm; marked tortuosity (hyperacute bend), aortic arch atheroma (especially if > 5
mm, protruding or ulcerated) or narrowing (especially with calcification and surface irregularities) of the abdominal or thoracic aorta, severe "unfolding" and tortuosity of the thoracic aorta, unless the patient qualifies for a transapical or other aortic or subclavian approaches;
- Iliofemoral vessel characteristics that would preclude safe placement of an introducer sheath such as severe obstructive calcification, severe tortuosity or small vessel size (applicable for transfemoral patients only), unless the patient qualifies for a transapical or other aortic approach.
We are requesting public comments on this proposed determination pursuant to section 1862(l) of the Social Security Act. We are specifically interested in public comments on the use of Coverage with Evidence Development (CED) in this decision. After considering the public comments, we will make a final determination and issue a final decision memorandum.
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology.
AATS – American Association for Thoracic Surgery
ACC – American College of Cardiology
ACCF – American College of Cardiology Foundation
AS – Aortic Stenosis
AVR – Aortic Valve Replacement
CV – Cardiovascular
CK – Creatine Kinase
EVAR – Endovascular Aneurysm Repair
LVEF – Left Ventricular Ejection Fraction
MB — Myocardial Band
MI – Myocardial Infarction
PAS – Post Approval Study
PCI – Percutaneous Intervention
PI – Primary Investigator
RCT – Randomized Controlled Trial
SCAI – Society for Cardiovascular Angiography and Interventions
STS – Society of Thoracic Surgeons
TAVI – Transcatheter Aortic Valve Implantation
TAVR – Transcatheter Aortic Valve Replacement
TEVAR – Thoracic Endovascular Aortic Repair
WHO – World Health Organization
The published literature uses both TAVR and TAVI to refer to the subject of this review. Readers may consider these terms to be interchangeable for the purposes of this memorandum.
The most common valvular abnormality in the United States is aortic stenosis (AS), with an incidence of approximately five of every 10,000 adults (Dewey 2008). As our population ages, AS prevalence will continue to increase. Aortic valve disease exists as a continuum, and aortic valvular abnormalities are often seen in older individuals as demonstrated by the Cardiovascular Health Study in which 26% of participants, men and women over the age of 65, had a degree of aortic sclerosis (Carabello 2009). Aortic sclerosis, which is an irregular valve thickening with no obstruction to ventricular blood outflow, is associated with age, sex, hypertension, smoking, diabetes, and serum LDL and lipoprotein levels and may progress to AS. The natural history in adults involves a long latent period where both morbidity and mortality are low. The progression of aortic stenosis to serious outflow obstruction causing sickness and death can be estimated, but much variability exists in the rate of progression, and it is not possible to predict the rate of progression in an individual patient. After the long latent period, symptoms of angina, syncope or heart failure can develop. On average, the survival is two to three years after symptoms develop, with a high risk of sudden death (Bonow 2008).
The most common cause of aortic stenosis in adults is calcification of the valve. This calcification progresses from the base of the cusps to the leaflets, and eventually causes a reduction in both leaflet motion and the effective valve area. This calcific disease is similar to atherosclerosis. Rheumatic AS disease, related to valvular infection, is less common. In young adults, congenital valve malformations are the more common cause for AS. The first sign of AS may be a murmur, detected during auscultation of the chest. If a murmur is detected, echocardiography may be indicated. Echocardiographic objective measurements include aortic jet velocity, mean pressure gradient and valve area. However, no single objective laboratory value defines severity or is the primary determinant of the need for valve replacement. Some patients with severe AS are asymptomatic, whereas others with only moderate stenosis develop symptoms. Therefore, therapeutic decisions are based mostly on the presence or absence of symptoms. For asymptomatic AS patients, the 2008 ACC/AHA guidelines recommend frequent monitoring for symptoms (which may be subtle), as well as disease progression (Bonow 2008). When patients develop symptoms thought to be due to AS, surgery is recommended. Symptomatic severe aortic stenosis carries a poor prognosis (Moat 2011).
Surgical aortic valve replacement (AVR) has been the gold standard for treatment in adults with severe symptomatic aortic stenosis and well-defined treatment guidelines exist (Dewey 2008). Until recently, surgical AVR has been the only effective treatment. In patients selected for isolated valve repair, the perioperative risk is low. Perioperative mortality in the Society of Thoracic Surgeons (STS) database is 3.0% to 4.0% for isolated AVR and 5.5% to 6.8% for AVR and coronary artery bypass graft (CABG) (Bonow 2008). Studies have shown that even in octogenarians AVR operative mortality was about 5-6%, with five year survival of 64-77% (Filsoufi 2008; ElBardissi 2011). Outcomes can vary based on surgical volume (Bonow 2008). However, risk can be increased for some patients (Moat 2011). Transcatheter aortic valve implantation (TAVI) was developed as an alternative to aortic valve replacement for populations that are thought to be at high risk for surgery.
Despite clear guidelines, excellent surgical outcomes, and high mortality of symptomatic valve disease, some patients do not receive necessary treatment. “Some patients with severe symptomatic aortic stenosis do not undergo aortic valve replacement despite demonstrated symptomatic and survival advantages and despite unequivocal guideline recommendations for surgical evaluation” (Bach 2009). Bach and colleagues estimate that one third of patients with severe AS are symptomatic but do not undergo surgical replacement, with the findings not limited to any specific practice environment. For many of these unoperated patients, objective ascertainment did not outwardly reject the possibility of surgery with apparent involvement of both physician and patient subjective decision-making. The conclusion has been drawn that some patients with severe symptomatic AS may be inappropriately denied access to potentially life-saving surgery (Bach 2009).
Technologic advancements have allowed for the delivery of heart valves via catheter as an alternative to open surgical valve replacement. The first in man studies were performed in 2002, and as such, TAVR is a relatively new procedure. TAVR treats the stenotic heart valve by displacing and functionally replacing the native aortic valve with a bioprosthetic valve delivered on a catheter via a percutaneous transarterial approach through a peripheral artery (e.g., the femoral artery), a transaortic approach through a limited sternotomy, or a transapical approach through a limited lower thoracotomy. Two devices, the SAPIEN and the CoreValve prostheses, are currently under post-market surveillance in Europe. The valve delivery system for these devices is similar, but the final step of implantation differs. The SAPIEN valve is a balloon-expandable bioprosthesis, whereas the CoreValve represents a self-expandable nitinol frame bioprosthesis. Proper technique with either is crucial. Though these implanted valves have been in use outside of the United States and sovereign registries exist to ascertain patient outcomes, none except for Moat and colleagues all-inclusive registry (with now two year outcomes) in the United Kingdom have yet reported significant numbers of consecutively enrolled patients with long-term follow-up. This is of great importance as the valve is expected to last the life of the patient (Moat 2011).
Postoperative complications lead to patient suffering, as well as increased burden. Therefore, it is important to identify patients who are at increased risk for surgical complications to guide future treatment decisions. Historically, this was a decision based on personal experience of the surgeon. To help in this decision and to provide reliable and accurate information for patients, a number of risk scoring systems have been developed. Saxton and Velanovich (2011) noted “the usefulness of the available scoring systems for accurately predicting postoperative complications is quite variable among different patient populations, indications for surgery, and surgical procedures performed.” This situation exists in large part because, although many morbidity and mortality risk factors for these scores have been extensively analyzed, considerable uncertainty remains regarding which patients will actually experience adverse outcomes. This is especially true in the elderly. Foremost among factors that have undergone investigation are patient age, comorbidities, physical examination findings and laboratory values (Saxton and Velanovich 2011). Recently, there has also been interest in more abstract concepts such as frailty and quality of life as risk predictors. Frailty is used to define older adults with impaired resistance to stressors due to decline in physiologic reserve, and is felt by some to better reflect biologic age as opposed to chronologic age (Afilalo 2011). Ultimately, however, any procedure is a risk-benefit decision. It is a critical endeavor to accurately determine such risk-benefit information for patient decision-making and empowerment.
III. History of Medicare Coverage
CMS does not have a national policy that addresses coverage of transcatheter aortic valve replacement (TAVR) or, as it is also known, transcatheter aortic valve implantation (TAVI).
Benefit Category
For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Social Security Act. Transcatheter aortic valve replacement (TAVR) falls under the benefit categories set forth in section §1861(b)(3) (inpatient hospital services), a part A benefit under §1812(a)(1), and §1861(s)(1) (physician services), a part B benefit. This may not be an exhaustive list of all applicable Medicare benefit categories for this item or service.
Current Request
On September 22, 2011, we received a formal complete written request from The Society of Thoracic Surgeons and The American College of Cardiology, submitted jointly. The request, available at http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id257.pdf, notes that the clinical outcomes reported in the pivotal trial were achieved when specific criteria were met.
Thus, we are asked to establish national Medicare coverage for TAVR with conditions of coverage, specifically when the procedure is
- "Performed in a specialized heart center utilizing a modified conventional cardiac laboratory or hybrid operating room that contains the specialized equipment necessary for the procedure;
- Managed using a multidisciplinary team using planned approach to co-management decision making as well as technical insertion of the device;
- Reported on using a joint STS-ACC TVT Registry."
The joint specialty society request recommends that CMS “include as a condition of coverage mandatory reporting of the procedures in an STS-ACC Transcatheter Valvular Therapy (TVT) Registry which would include long term follow-up using CMS data.”
IV. Timeline of Recent Activities
| Date |
Action |
| September 28, 2011 |
CMS accepts formal request from the Society of Thoracic Surgeons (STS) and American College of Cardiology (ACC), and initiates this national coverage analysis for transcatheter aortic valve replacement. The initial 30-day public comment period begins. |
| October 28, 2011 |
Initial 30-day public comment period closes. |
V. FDA Status
On November 2, 2011 the Food and Drug Administration (FDA) approved the first TAVR device for marketing in the United States. The Edwards’ Sapien Transcatheter Heart Valve (THV) was approved “for transfemoral delivery in patients with severe symptomatic native aortic valve stenosis who have been determined by a cardiac surgeon to be inoperable for open aortic valve replacement and in whom existing co-morbidities would not preclude the expected benefit from correction of the aortic stenosis” (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=P100041).
FDA approval (http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100041a.pdf) includes a statement recommending specific training and experience for practitioners to use the device, as well as continued clinical study and data submission to the ACC STS TVT Registry.
VI. General Methodological Principles
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. The critical appraisal of the evidence enables us to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for beneficiaries. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
A detailed account of the methodological principles of study design that the Agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A.
Public commenters sometimes cite the published clinical evidence and provide CMS with useful information. Public comments that provide information based on unpublished evidence, such as the results of individual practitioners or patients, are less rigorous and, therefore, less useful for making a coverage determination. CMS uses the initial comment period to inform its proposed decision. CMS responds in detail to the public comments that were received in response to the proposed decision when it issues the final decision memorandum.
VII. Evidence
A. Introduction
This presentation of evidence primarily focuses upon whether the pivotal PARTNER randomized controlled trials (RCTs) are adequate to draw conclusions about health outcomes for TAVR, as well as whether the body of evidence is generalizable to the Medicare population. The evidence CMS examines has as its focus health outcomes, i.e., the benefits and harms of a particular treatment. Key outcomes of interest to CMS are periprocedural and long-term risk of stroke or death, as well as health-related quality of life and function post-TAVR. Independently assessed, validated instruments are most heavily weighted.
We summarize the evidence relating to the treatment of symptomatic aortic stenosis with the transcatheter aortic valve which includes the FDA premarket application clinical trial that was divided into two groups (PARTNER A and PARTNER B) based upon patient selection criteria. Previous case series studies have also been reviewed, but add little to the conclusions of the PARTNER studies as they are either non-consecutive or small. In treatment of symptomatic aortic stenosis, the primary focus is reduction in symptoms (chest pain, shortness of breath, fatigue and weakness), cardiovascular events (heart failure, stroke, myocardial infarction, arrhythmia) and mortality (cardiovascular mortality), as well as improvement in quality of life (QOL) and function.
Study endpoints should be clearly defined a priori to both improve the quality of clinical research and so as to allow comparison between clinical trials. For transcatheter aortic valve implantation (TAVI) clinical trials, a report was published proposing standardized consensus definitions for important clinical endpoints (Leon 2011). In this consensus document, the following outcomes were given clinically relevant definitions:
- Cardiovascular mortality,
- Myocardial infarction,
- Stroke,
- Bleeding,
- Acute kidney injury,
- Vascular access site and access-related complications,
- Potential failure modes of prosthetic valve dysfunction.
Functional outcome measures for aortic stenosis include the New York Heart Association (NYHA) classification (I-IV), the six minute walk test (6MWT), the fifty meter walk test, and the modified Rankin Scale (mRS). The NYHA classification is a subjective symptom measure. The six minute walk test is (as the name describes) a standardized approach, which can be effort dependent, and the 6MWT is similar to the fifty meter walk test. The mRS is a measure of stroke disability and reportedly provides a better impression of whether patients are able to look after themselves than activities of daily living (ADL) scores (Van Swieten 1988). The mRS has six classifications: 0 = no symptoms; 1 = no significant disability; 2 = slight disability; 3 = moderate disability; 4 = moderately severe disability, unable to walk without assistance, and unable to attend to own bodily needs without assistance; 5 = severe disability, including the bedridden, incontinent, and requiring constant nursing care and attention.
Quality of life is important to Medicare beneficiaries and can weigh heavily in patients’ decision-making. Therefore, valid and reliable measurement is important to inform patients. Quality of life (QOL) measures can be disease specific or general. Disease specific measures used in TAVR trials have included the Kansas City Cardiomyopathy Questionnaire (KCCQ), which is a 23-item questionnaire for assessment of disability and quality of life impairment due to congestive heart failure. Other heart failure assessments include the Minnesota Living with Heart Failure. Generic QOL assessments included the SF-36, SF-12, PROMISE, and the EuroQOL for population comparisons. There are advantages and disadvantages to each tool, and the end use can help with tool choice, i.e., disease specific to measure within the population, and generic for a broad population comparison. Physiologic measures such as hemodynamic measurement by echocardiography are also used but their relationship to clinical outcomes is less clear.
B. Discussion of Evidence
1. Questions:
The development of an assessment in support of Medicare coverage decisions is based on the same general question for almost all national coverage analyses (NCAs): "Is the evidence sufficient to conclude that the application of the item or service under study will improve health outcomes for Medicare patients?" For this NCD, the questions of interest are:
- Is the evidence adequate to conclude that transcatheter aortic valve replacement improves health outcomes for Medicare beneficiaries with severe symptomatic aortic stenosis who are not candidates for surgical aortic valve replacement?
- Is the evidence adequate to conclude that transcatheter aortic valve replacement improves health outcomes for high surgical risk Medicare beneficiaries with severe symptomatic aortic stenosis who are candidates for surgical aortic valve replacement?
If the answer to either or both of the questions above is positive, is the available evidence adequate to identify the characteristics of the patient, practitioner or facility that predict which beneficiaries are more likely to experience overall benefit or harm from TAVR?
2. External Technology Assessment (TA)
CMS did not commission an external TA for this NCA, but an October 12, 2011 Belgian health technology assessment (Neyt 2011) and one interventional procedure overview/review (NICE 2011) were identified which analyzed the PARTNER study.
Belgian Health Technology Assessment (2011)
Neyt M, Van Brabandt H, Van de Sande S, et al. Health technology assessment. Transcatheter aortic valve implantation (TAVI): a health technology assessment update. Health Technology Assessment (HTA) 2011 Brussels: Belgian Health Care Knowledge Centre (KCE). KCE Reports 163C. D/2011/10.273/48 Available online: http://kce.fgov.be/sites/default/files/page_documents/kce_163c_tavi_update.pdf
The 2011 Belgian Health Technology Assessment made the following key points about the PARTNER trial in general (as well as for both cohorts) and also provided critical analysis regarding particularly the PARTNER trial’s internal validity and physicians’ preferences:
General Remarks
- “TAVI is a highly invasive and challenging procedure addressing elderly people in poor general condition. The procedure takes on average over 4 hours (skin-to-skin time 2 to 3 hours). It involves prolonged general anaesthesia, the administration of contrast media, and trans-oesophageal echocardiography. It is complicated with hemorrhagic vascular adverse events in more than 50% of patients.
- Differentiating “patients who cannot undergo surgery” (PARTNER Cohort B) from surgical high-risk patients” (Cohort A) essentially relies on the clinical feeling of the physicians involved.
- The treatment effect of TAVI may be overestimated in PARTNER because of methodological concerns and a potential impact of conflicts of interest. Long term outcomes related to a residual aortic regurgitation after TAVI, and the long term durability of the prosthesis remain unknown.”
PARTNER Cohort A
- “In patients with aortic stenosis who are at very high surgical risk, TAVI and surgery are associated with a similar mortality rate at 30 days and 1 year and produce similar improvements in cardiac symptoms.
- The abovementioned observation dissolves our initial safety concerns of TAVI, but the approximate doubling in the rate of stroke 1 year after TAVI (8.3%) compared to surgery (4.3%) remains a concern.
- The 30-day mortality rate of TAVI observed in Cohort A of the PARTNER trial is the lowest ever reported in a TAVI study although most of the participating centres had no previous experience with TAVI.”
PARTNER Cohort B
- “The PARTNER trial does not allow [us] to assess the performance of trans-apical TAVI in inoperable patients.
- In patients with severe aortic stenosis who are no [sic] candidates for surgery, TAVI significantly reduces the rate of death from any cause (ARR 20% at 1 year) as compared with standard therapy.
- In the Continued Access population (n = 90), TAVI had an absolute 12.7% higher mortality at 1 year as compared with standard therapy.
- Standard therapy included a balloon aortic valvuloplasty in most patients, a procedure considered as a palliative measure that has never been shown to be more effective than medical treatment.
- Stroke rate at 1 year was twice as high in TAVI patients compared to standard therapy (10.6% vs. 4.5%).
- In Cohort B, patients with prohibitive anatomical conditions were unevenly represented in both study groups. Subgroup analysis of those patients showed a more favourable effect of TAVI at 30 days (4.4% absolute survival difference) and after 1 year (8.8% absolute difference) compared to patients with medical prohibitive conditions.”
Internal Validity
- “Critical analysis of the methodology used in the PARTNER study indicates a rather high risk of bias, mainly in Cohort B.
- The unequal distribution of the basic characteristics between the study groups, to the advantage of TAVI, raises questions as to whether patient randomisation proceeded correctly. The randomisation procedure is only described in brief in the study protocol and our requests for further explanation from the study sponsor did not provide additional clarity. The fact that the main author of the study had significant financial interests in demonstrating the efficacy of TAVI raises eyebrows.
- Furthermore, the unexpected results of the Continued Access study that were conflicting with those of the pivotal trial raise questions.
- The so-called “standard therapy” involved an aortic balloon valvuloplasty in 84% of the patients in Cohort B. According to international practice guidelines, this form of treatment can sometimes be justified as an approach to treat aortic stenosis in the extreme elderly, but is anything but the standard. It is actually also a highly invasive technique with its own inherent severe risks. Its added value with respect to strictly medical treatments has never been demonstrated.
- In the elderly with severe aortic valve stenosis and severe co-morbidities, any procedure performed on the aortic valve should be considered as a palliative therapy. Such treatment decisions are determined by the question as to whether the quality of life of the patient in question, with his/her additional severe non-cardiac problems, can be expected to improve. This was not sufficiently demonstrated in the PARTNER study.”
Physicians’ Preferences
- “A physician’s performance in estimating the operative risk of a patient with aortic stenosis and significant co-morbidities has not been clearly established and may be subject to bias. In this respect, ethical questions come into play. Depending on the physicians’ preferences, less sick patients may be treated by TAVI although they could reasonably have open AVR. On the other hand, some patients may be offered TAVI although their co-morbidities preclude any significant improvement in their quality of life with a correction of the aortic stenosis. In a recent comment, the FDA deplores that whereas the PARTNER trial protocol defined patients who should not have surgery due to extensive co-morbidities, it did not actively consider patients who should not have TAVI.”
Transcatheter Aortic Valve Implantation for Aortic Stenosis (NICE July 25, 2011)
National Institute for Health and Clinical Excellence (NICE). Interventional procedures programme. Interventional procedure overview of transcatheter aortic valve implantation for aortic stenosis. 25 July 2011. http://www.nice.org.uk/nicemedia/live/11914/55669/55669.pdf
The National Institute for Health and Clinical Excellence (NICE) reviewed its 2008 guidance and provided an overview of transcatheter aortic valve implantation for aortic stenosis (April 2011). NICE sought additional consultation comments (July 2011) and is reassessing the procedure prior to issuing new interventional procedure guidance which is currently pending publication.
At the time of the April 2011 overview, NICE noted that in the U.K., TAVI is usually performed in patients who are very ill and are therefore inappropriate for conventional surgery, so TAVI is usually palliative in intent. Long-term data are lacking, with maximum median follow-up of 3.7 years. NICE also noted that “it is difficult to compare outcomes in non-randomized comparative studies since patients who are selected for TAVI are likely to be more ill and more likely to suffer complications or die.” Additionally, “Advisers stated that the procedure should only be performed by interventional cardiologists or cardiac surgeons with outstanding interventional experience and skills. The importance of a multidisciplinary team of surgeons and cardiologists when performing was highlighted.”
3. Internal Technology Assessment
CMS searched PubMed from 2000-2011 for randomized controlled trials (RCTs), substudies of such RCTs regarding quality of life; technology assessments, systematic reviews and clinical guidelines which featured or included the pivotal PARTNER trial; device complications; plus prospective registries of consecutively enrolled patients reporting long-term outcomes, with keywords including symptomatic, severe aortic stenosis, transcatheter, aortic valve, implantation and/or replacement. We additionally searched for recent studies and reviews of frailty. To define the standard therapy to which TAVR has been compared in published clinical trials, we include recent reviews of open surgical and minimally invasive aortic valve replacement in the Medicare population. Studies must have been published in peer-reviewed English language journals. Abstracts, voluntary registries, and studies with less than 50 patients (unless reporting a significant adverse event not published elsewhere) were excluded. The literature search was limited to the English language and specific to the human population, but included studies conducted in all countries, including the United States. Public access information from the FDA website was also used.
Evidence Summary
Standard Open and Minimally Invasive Surgical AVR
Melby S, Zierer A, Kaiser S, et al. Aortic valve replacement in octogenarians: risk factors for early and late mortality. Annals of Thoracic Surgery 2007 May;83(5):1651-1656; discussion 1656-1657.
Melby and colleagues from the Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis concluded: “Patients aged 80 years and older who undergo AVR have acceptable short-term and long-term survival regardless of NYHA status. Concomitant CABG [coronary artery bypass grafting] improved operative and long-term survival in this population. Despite their increased age, aggressive surgical treatment is warranted for most patients.”
Filsoufi F, Rahmanian P, Castillo J, et al. Excellent early and late outcomes of aortic valve replacement in people aged 80 and older. Journal of the American Geriatrics Society 2008 February;56(2):255-261.
Filsoufi and colleagues from Mount Sinai School of Medicine in New York concluded: “Excellent results after AVR can be expected in patients aged 80 and older, with minimal increase in postoperative mortality and acceptable postoperative morbidity. Respiratory failure is the main postoperative complication in patients aged 80 and older. Recent advances in operative techniques and perioperative management have contributed to better surgical outcomes in these patients than found in historical reports.”
Thourani V, Myung R, Kilgo P, et al. Long-term outcomes after isolated aortic valve replacement in octogenarians: a modern perspective. Annals of Thoracic Surgery 2008 November;86(5):1458-1464; discussion 1464-1465.
Thourani and colleagues from the Emory University School of Medicine in Atlanta concluded: “In the modern era, octogenarians have acceptable short- and long-term results after open AVR. Comparisons of less invasive techniques for AVR should rely on outcomes based in the modern era and decisions regarding surgical intervention in patients requiring AVR should not be based on age alone.”
ElBardissi A, Shekar P, Couper G, et al. Minimally invasive aortic valve replacement in octogenarian, high-risk, transcatheter aortic valve implantation candidates. Journal of Thoracic and Cardiovascular Surgery 2011 February;141(2):328-335.
ElBardissi and colleagues from the Brigham and Women's Hospital and the Harvard Medical School in Boston concluded: “Patients thought to be high-risk candidates for surgical aortic valve replacement have excellent outcomes after minimally invasive surgery with long-term survival that is no different than that of an age- and gender-matched U.S. population. These data provide a benchmark against which outcomes of transcatheter aortic valve implantation could be compared.”
Randomized Controlled Trials of Transcatheter AVR
Leon M, Smith C, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New England Journal of Medicine 2010 October 21;363(17):1597-1607.
Smith C, Leon M, Mack M, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. New England Journal of Medicine 2011 June 9;364(23):2187-2198.
The PARTNER study incorporated two parallel prospective, unblinded, randomized, active-treatment controlled, multi-center pivotal trials evaluating the safety and effectiveness of transcatheter aortic valve replacement, via transfemoral or transapical (Cohort A only) delivery, in a stratified population of high risk (Cohort A) or inoperable (Cohort B) patients.
Because the study enrolled two distinct populations, two cohorts were separately-powered and analyzed. As depicted in Figure 1, an initial stratification based on operability for aortic valve replacement (AVR) surgery was used to assign patients to either Cohort A or B. Assignment to cohorts was followed by determination of vascular access for transfemoral delivery. Patients who were considered high surgical risk and eligible for transfemoral access were stratified into Cohort A and randomized to treatment (transfemoral AVR) or control (surgical AVR). Cohort A patients who were not eligible for transfemoral access were evaluated as candidates for transapical delivery and, if appropriate, randomized to treatment (transapical AVR) or control (surgical AVR). Those patients who were considered non-surgical candidates were stratified into Cohort B and randomized to treatment (transfemoral AVR) or control (“standard” therapy). Those assigned to Cohort B who did not meet the criteria for transfemoral delivery were not enrolled in the study because the sponsor declined to have a transapical arm in Cohort B (PARTNER trial protocol 2009; FDA Executive Summary 2011).
Figure 1. PARTNER Trial Enrollment Diagram (FDA Executive Summary 2011)
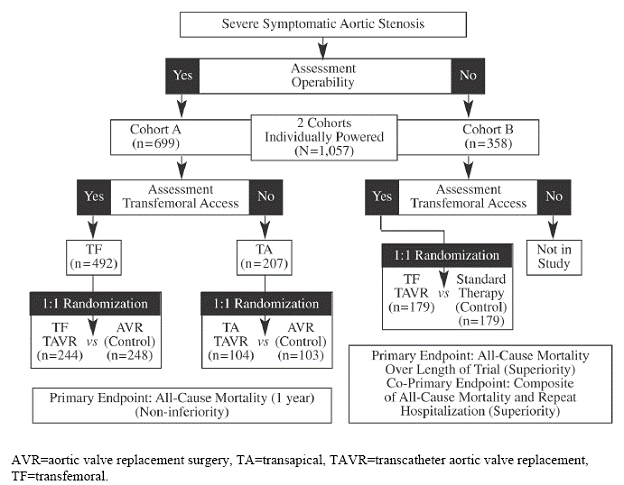
“The “standard” therapy control group predominantly consisted of subjects receiving BAV (78.2%); other patients received medical therapy alone (7.9%), AVR (6.1%), apical-aortic conduits (3.3%), or TAVR outside of the U.S. (2.2%)” (FDA Executive Summary 2011).
Candidates for the pivotal PARTNER trial were highly selected (1057 of 3105 (34%) patients who were screened at all the study centers underwent randomization) and each candidate must have met all of the following inclusion/exclusion criteria:
PARTNER Cohort A Inclusion Criteria
“All candidates for Cohort A of this study must meet all of the following Inclusion criteria:
- Patients must have co-morbidities such that the surgeon and cardiologist Co-PIs concur that the predicted risk of operative mortality is ≥ 15% and/or a minimum STS score of 10. A candidate who does not meet the STS score criteria of ≥ 10 can be included in the study if a peer review by at least two surgeon investigators (not including the enrolling surgeon) concludes and documents that the patient’s predicted risk of operative mortality is ≥ 15%. The surgeon's assessment of operative comorbidities not captured by the STS score must be documented in the study case report form as well as in the patient medical record.
- Patient has senile degenerative aortic valve stenosis with echocardiographically derived criteria: mean gradient > 40 mmHg or jet velocity greater than 4.0 m/s or an initial aortic valve area (AVA) of < 0.8 cm2 (indexed EOA < 0.5 cm2/m2). (Qualifying AVA baseline measurement must be within 45 days prior to randomization).
- Patient is symptomatic from his/her aortic valve stenosis, as demonstrated by NYHA Functional Class II or greater.
- The subject or the subject’s legal representative has been informed of the nature of the study, agrees to its provisions and has provided written informed consent as approved by the Institutional Review Board (IRB) of the respective clinical site.
- The subject and the treating physician agree that the subject will return for all required post-procedure follow-up visits.”
PARTNER Cohort B Inclusion Criteria
“All candidates for Cohort B of this study must meet # 2, 3, 4, 5 of the above criteria, and
- The subject, after formal consults by a cardiologist and two cardiovascular surgeons agree that medical factors preclude operation, based on a conclusion that the probability of death or serious, irreversible morbidity exceeds the probability of meaningful improvement. Specifically, the probability of death or serious, irreversible morbidity should exceed 50%. The surgeons' consult notes shall specify the medical or anatomic factors leading to that conclusion and include a printout of the calculation of the STS score to additionally identify the risks in these patients.”
PARTNER (Cohort A and B) Exclusion Criteria
“Candidates will be excluded from the study if any of the following conditions are present:
- Evidence of an acute myocardial infarction ≤ 1month before the intended treatment (defined as: Q wave MI, or non-Q wave MI with total CK elevation of CK-MB ≥ twice normal in the presence of MB elevation and/or troponin level elevation (WHO definition).
- Aortic valve is a congenital unicuspid or congenital bicuspid valve, or is non-calcified.
- Mixed aortic valve disease (aortic stenosis and aortic regurgitation with predominant aortic regurgitation >3+).
- Any therapeutic invasive cardiac procedure performed within 30 days of the index procedure, (or 6 months if the procedure was a drug eluting coronary stent implantation).
- Pre-existing prosthetic heart valve in any position, prosthetic ring, severe mitral annular calcification (MAC), severe (greater than 3+) mitral insufficiency, or Gorelin syndrome
- Blood dyscrasias as defined: leukopenia (WBC < 3000 mm3), acute anemia (Hb < 9 mg%), thrombocytopenia (platelet count < 50,000 cells/mm3), history of bleeding diathesis or coagulopathy.
- Untreated clinically significant coronary artery disease requiring revascularization.
- Hemodynamic instability requiring inotropic support or mechanical heart assistance.
- Need for emergency surgery for any reason.
- Hypertrophic cardiomyopathy with or without obstruction (HOCM).
- Severe ventricular dysfunction with LVEF < 20.
- Echocardiographic evidence of intracardiac mass, thrombus or vegetation.
- Active peptic ulcer or upper GI bleeding within the prior 3 months.
- A known hypersensitivity or contraindication to aspirin, heparin, ticlopidine (Ticlid), or clopidogrel (Plavix), or sensitivity to contrast media, which cannot be adequately premedicated.
- Native aortic annulus size < 18mm or > 25mm as measured by echocardiogram.
- Patient has been offered surgery but has refused surgery.
- Recent (within 6 months) cerebrovascular accident (CVA) or a transient ischemic attack (TIA).
- Renal insufficiency (creatinine > 3.0) and/or end stage renal disease requiring chronic dialysis.
- Life expectancy < 12 months due to non-cardiac co-morbid conditions.
- Significant aortic disease, including abdominal aortic or thoracic aneurysm defined as maximal luminal diameter 5cm or greater; marked tortuosity (hyperacute bend), aortic arch atheroma (especially if thick [> 5 mm], protruding or ulcerated) or narrowing (especially with calcification and surface irregularities) of the abdominal or thoracic aorta, severe “unfolding” and tortuosity of the thoracic aorta (applicable for transfemoral patients only).
- Iliofemoral vessel characteristics that would preclude safe placement of 22F or 24F introducer sheath such as severe obstructive calcification, severe tortuosity or vessels size less than 7 mm in diameter (applicable for transfemoral patients only).
- Currently participating in an investigational drug or another device study. [Note: Trials requiring extended follow-up for products that were investigational, but have since become commercially available, are not considered investigational trials].
- Active bacterial endocarditis or other active infections.
- Bulky calcified aortic valve leaflets in close proximity to coronary ostia.”
(http://www.nejm.org/doi/suppl/10.1056/NEJMoa1008232/suppl_file/nejmoa1008232_protocol.pdf)
“Changes in the protocol were made after this unblinded study started enrollment, the most significant of which was the addition of a co-primary composite endpoint of mortality and hospitalization. The 6-minute walk test endpoint was also added after the start of the trial. The protocol was fully approved in March 2009 (Version 3.2) coincident with completion of enrollment into the Cohort B study, and approval to begin a Continued Access study. At the onset, the Cohort B continued access study protocol was the same as the randomized PARTNER study until Cohort A enrollment was completed. In August 2009, enrollment into the Cohort A study was completed, and the Continued Access study was expanded to allow enrollment of Cohort A subjects in a non-randomized protocol. Randomization for the Cohort B group was also discontinued at that time” (FDA Executive Summary 2011).
Figure 2. Timeline of PARTNER trial and the Continued Access study (Neyt 2011)
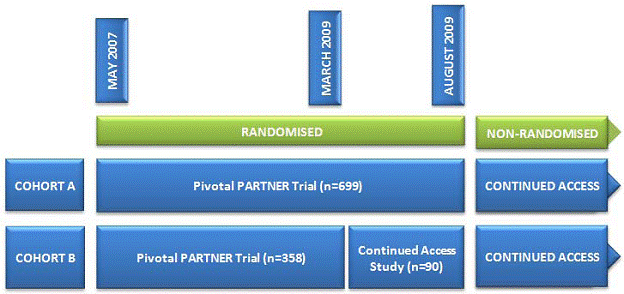
“Enrollment in the nonrandomised Continued Access cohort is ongoing. As of November 1, 2010, 160 nonrandomized patients have been enrolled” (Belgian HTA 2011).
PARTNER Cohort A (2011)
Smith C, Leon M, Mack M, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. New England Journal of Medicine 2011 June 9;364(23):2187-2198.
At 25 centers in the United States (22 centers), Canada (2 centers), and Germany (1 center), 699 high-risk patients (348 TAVI versus 351 surgical aortic valve replacement) with severe aortic stenosis and cardiac symptoms (NYHA class II function or worse) – who were considered to be candidates for surgery despite the fact that they were at high surgical risk – were assigned to either transcatheter aortic valve replacement with a balloon-expandable bovine pericardial valve (either transfemoral or transapical approach) or surgical aortic valve replacement in an industry sponsored trial. As outlined in Cohort A’s inclusion criteria, “severe aortic stenosis was defined as an aortic-valve area less than 0.8 cm2 plus either a mean gradient of at least 40mm Hg or a peak velocity of at least 4.0 m per second.” High risk for operative complications or death determination was made by at least two surgeons at each center, using as a guideline a score of ≥ 10% on the Society for Thoracic Surgeons (STS) risk model or due to presence of coexisting conditions associated with ≥ 15% predicted risk of death by 30 days after surgery.
Mean age was 83.6 years for the TAVI group and 84.5 years for the surgical group; and females comprised 42.2% in the TAVI group and 43.3% in the surgical control group. Baseline characteristics were comparable between groups. Extensive exclusion criteria for both Cohort A and Cohort B of the PARTNER trial included: bicuspid or non-calcified aortic valve, coronary artery disease requiring revascularization, left ventricular ejection fraction (LVEF) < 20%, aortic annulus < 18mm or > 25 mm, severe mitral or aortic regurgitation, recent neurological event, and severe renal insufficiency. Patients in the transcatheter group underwent either transfemoral or transapical placement of the aortic valve, on the basis of whether peripheral arteries could accommodate the sheath. Randomization was achieved with the use of computer-generated randomized blocks at each site and for each subgroup. The primary end point was death from any cause at one year in the intention-to-treat population. The primary hypothesis was that transcatheter replacement is not inferior to surgical replacement. All patients were followed for one year, starting during the index hospitalization, 30 days, six months, one year, and then yearly. Crossover between the two groups was permitted only when findings during the assigned procedure suggested the alternate treatment. Pre-specified secondary end points included:
- Death from cardiovascular causes;
- NYHA functional class;
- Repeat hospitalization because of valve- or procedure-related clinical deterioration;
- Myocardial infarction;
- Stroke;
- Acute kidney injury;
- Vascular complications;
- Bleeding;
- 6-minute walk distance;
- Valve performance (assessed with echocardiography).
“In a retrospective analysis of neurologic events adjudicated by the clinical events committee, major stroke was defined by a score of at least 2 on the modified Rankin scale (which ranges from 0 to 6, with higher scores indicating greater disability).” A priori, the investigators determined sample size between the two groups, with the design to demonstrate non-inferiority. A sample of 650 patients would provide a power of at least 85% to show non-inferiority of the primary end point, assuming a 1-year death rate of 29% in the transcatheter group and 32% in the surgical group. Non-inferiority would be established if the upper limit of the one-sided 95% confidence interval for the between-group difference in mortality was less than 7.5 percentage points, with alpha of 0.05. Other sample size computations were done, such as considering transfemoral placement independently. Fisher’s exact test was used for categorical variables and continuous variables were compared with the use of Student’s t-test. Time-to-event analyses, based on available data, were done with the use of Kaplan-Meier estimates and compared between groups with the use of the log-rank test. A test for interactions was performed. All analyses were intention-to-treat (not as treated).
Four patients died during the procedure (three in the experimental group and one in the control). Sixteen patients in the TAVI group (4.6%) received conventional surgical repair. One patient in the surgical group underwent transapical replacement. Multiple transcatheter valves (> 2) were implanted in seven patients due to difficulties, three of these patients died. Among seven other patients with similar difficulties, transcatheter placement was aborted in two patients, and was converted to open surgery in five patients. Late interventions in the TAVI group included another procedure (valvuloplasty) for paravalvular regurgitation in two patients and conversion to transapical placement in one patient. Additionally, patients in the TAVI group received heparin during the procedures and aspirin and clopidogrel for six months after the procedure.
Serious events were adjudicated by an independent committee, and a complete account of the “Clinical Outcomes at 30 Days and 1 Year in the Intention-to-Treat Population” for patients in Cohort A (Table 2 from Smith 2011) can be found in Appendix B at the end of this proposed decision memorandum. Additional outcomes included:
Patient functional measures
- At 30 days, more patients in the experimental group had a reduction in symptoms to NYHA class II or lower.
- At 30 days, for patients who were able to perform the 6-minute walk test, patients in the transcatheter group walked farther than those in the surgical group (NICE 2011).
- At one year, the earlier between-group differences were not evident.
Echocardiographic data
- Aortic-valve gradients and area improved significantly after the two procedures at both 30 days and one year, as expected.
- At one year, transcatheter replacement was slightly superior to surgical replacement with respect to the mean aortic-valve gradient and mean valve area.
- Moderate or severe paravalvular regurgitation was more frequent in the transcatheter group than in the surgical group at 30 days (12.2% vs. 0.9%) and at one year (6.8% vs. 1.9%) (P < 0.001 for both comparisons).
The authors concluded: “In high-risk patients with severe aortic stenosis, transcatheter and surgical procedures for aortic-valve replacement were associated with similar rates of survival at 1 year, although there were important differences in periprocedural risks.”
PARTNER Cohort B (2010)
Leon M, Smith C, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. New England Journal of Medicine 2010 October 21;363(17):1597-1607.
From 25 participating centers in the United States (21 centers), Canada (3 centers), and Germany (1 center), 358 high-risk patients (179 TAVI versus 179 control patients) with severe aortic stenosis and cardiac symptoms – who were not considered to be suitable for surgery – were enrolled at 21 sites (17 in the United States) and were randomly assigned to either transcatheter aortic valve replacement (AVR) with a balloon-expandable bovine pericardial valve (either a transfemoral or a transapical approach) or standard therapy (including balloon aortic valvuloplasty but not conventional surgery) in an industry sponsored trial. As outlined in Cohort B’s inclusion criteria, severe aortic stenosis was defined as an aortic-valve area less than 0.8 cm2 and either a mean gradient of at least 40mm Hg or a peak velocity of at least 4.0m per second. These patients were considered not to be suitable candidates for surgery due to the presence of coexisting conditions that could be associated with a predicted probability of 50% or more of either death by 30 days after surgery or a serious irreversible condition, as agreed upon by at least two surgeons.
Mean age was 83.1 years for the TAVI group and 83.2 years for the surgical group, and females comprised 54.1% in the TAVI group and 54.1% in the standard therapy control group. Baseline characteristics were not balanced and included several between-group differences that were statistically significant (p < 0.05):
- Lower logistic EuroSCORE in the TAVI group (p = 0.04);
- More patients with COPD in the control group (p = 0.04);
- More patients with atrial fibrillation in the control group (p = 0.04); and
- More patients with an extensively calcified aorta in the TAVI group (p = 0.05).
Exclusion criteria included: bicuspid or non-calcified aortic valve, acute myocardial infarction, substantial coronary artery disease requiring revascularization, LVEF < 20%, aortic annulus< 18mm or > 25 mm, severe mitral or aortic regurgitation, recent neurological event, and severe renal insufficiency. Patients in the transcatheter group underwent transfemoral placement of the valve. Randomization was achieved with the use of computer-generated randomized blocks at each site and for each subgroup. The primary end point was death from any cause at 1 year in the intention-to-treat population. “The co-primary end point was the rate of a hierarchical composite of the time to death from any cause or the time to the first occurrence of repeat hospitalization (after the index procedure) due to valve-related or procedure-related clinical deterioration.” The primary hypothesis was that transcatheter replacement is not inferior to standard therapy. All patients were followed for one year, starting during index hospitalization, 30 days, six months, one year, and then yearly. Crossover was not permitted. Pre-specified secondary end points included:
- Death from cardiovascular causes;
- NYHA functional class;
- Repeat hospitalization because of valve- or procedure-related clinical deterioration;
- Myocardial infarction;
- Stroke;
- Acute kidney injury;
- Vascular complications;
- Bleeding;
- 6-minute walk distance;
- Valve performance (assessed with echocardiography).
A priori, the investigators determined sample size between the two groups, with the design to demonstrate superiority. A sample of 350 patients would provide a power of at least 85% to show superiority of the primary end point, assuming a one year death rate of 37.5% in the transcatheter group and 25% in the control group. The analysis of the co-primary endpoint was performed using a nonparametric method. The sample size of 350 patients with a power of 95% was estimated on the basis of the co-primary endpoint. Fisher’s exact test was used for categorical variables and continuous variables were compared with the use of Student’s t-test. Time-to-event analyses, based on available data, were done with the use of Kaplan-Meier estimates and compared between groups with the use of the log-rank test. A two-sided alpha level of 0.05 was used for all superiority testing. All analyses were intention-to-treat (not as treated).
Two patients randomized to TAVI died before receiving the intervention. Four patients in the TAVI group did not receive a valve due to technical difficulties. Despite being categorized as unsuitable for surgery, twelve patients underwent AVR (conventional surgery), another five underwent two procedures (placement of a conduit from the left ventricular apex to the descending aorta plus AVR), and four underwent TAVI at a non-participating site outside the United States. Three patients in the TAVI group underwent repeat TAVI to treat clinically significant aortic regurgitation. All patients in the TAVI group received heparin during the procedures, and aspirin and clopidogrel for six months after the procedure.
Serious events were adjudicated by an independent committee, and a full account of the “Clinical Outcomes at 30 Days and 1 Year” for patients in Cohort B (Table 2 from Leon 2010) can be found in Appendix B at the end of this proposed decision memorandum. Additional outcomes included:
Patient functional measures:
- At one year, 74.8% of surviving patients with TAVI as compared to 42.0% or the surviving patients with standard therapy had a reduction in symptoms to NYHA class II or lower.
- At one year, of the subgroup of patients able to perform the 6-minute walk, an analysis showed that there was significant improvement after TAVI and no change after standard therapy.
Echocardiographic data:
- At one year, the improvement in aortic-valve area and mean valve gradient was maintained.
- Moderate or severe paravalvular regurgitation was present in 11.8% of the patients in the TAVI group at 30 days and in 10.5% at one year. The incidence of moderate or severe transvalvular aortic regurgitation was 1.3% at 30 days and 4.2% at one year among patients in the TAVI group, as compared to 16.9% and 15.2% in the control group where the procedure for some was balloon valvuloplasty.
The authors concluded: “In patients with severe aortic stenosis who were not suitable candidates for surgery, TAVI, as compared with standard therapy, significantly reduced the rates of death from any cause, the composite end point of death from any cause or repeat hospitalization, and cardiac symptoms, despite the higher incidence of major strokes and major vascular events.”
Quality of Life
Reynolds M, Magnuson E, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 2011 November 1;124(18):1964-1972.
Reynolds and colleagues performed a prospective health-related quality of life (HRQOL) substudy among patients enrolled in the PARTNER trial. In this publication they presented only the results of the PARTNER trial’s Cohort B – those patients who were not considered candidates for surgical valve replacement and who were therefore randomized to either TAVR or the standard therapy control group. In the PARTNER trial, HRQOL was assessed at baseline and then at one, six, and 12 months with the Kansas City Cardiomyopathy Questionnaire (KCCQ) and the 12-item Short Form 12 General Health Survey (SF-12). The KCCQ has undergone validation in heart failure patients, and its summary score has a range of 1-100, with higher being a better score. At baseline between treatment groups, the KCCQ overall summary (including symptoms, physical limitation, social limitation, and quality of life) was not different statistically, nor was either of the two components of the SF-12 (physical and mental). Baseline scores for the KCCQ and SF-12 were low and subsequently improved in both groups, though the improvement was greater in the TAVR group as compared to controls at all measured time points, with both clinical and statistical difference. The authors concluded: “Among inoperable patients with severe aortic stenosis, compared with standard care, TAVR resulted in significant improvements in health-related quality of life that were maintained for at least 1 year.”
Observational Studies: Long-Term Outcomes
Moat M, Ludman P, Belder M, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis. The U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) registry. Journal of the American College of Cardiology 2011 November 8;58(20):2130-2138.
The United Kingdom (U.K.) established an all-inclusive transcatheter aortic valve implantation registry to report outcomes of all TAVI procedures performed within that country. A total of 25 centers throughout England and Wales developed active TAVI programs between January 2007 and December 2009, and data were collected prospectively on 870 patients undergoing 877 TAVI procedures up until December 31, 2009. Two technologies were available to these units: the Medtronic CoreValve system and the Edwards SAPIEN valve. Since there are few data on outcomes beyond one year, Moat and colleagues’ (2011) publication was an attempt to address outcomes to date. Mortality tracking was achieved in 100% of patients with survival status reported as of December 12, 2010; and follow-up ranged from 11 months to 46 months.
The authors reported that “Survival at 30 days was 92.9%, and it was 78.6% and 73.7% at 1 year and 2 years, respectively. There was a marked attrition in survival between 30 days and 1 year. In a univariate model, survival was significantly adversely affected by renal dysfunction, the presence of coronary artery disease, and a nontransfemoral approach; whereas left ventricular function (ejection fraction < 30%), the presence of moderate/severe aortic regurgitation, and chronic obstructive pulmonary disease remained the only independent predictors of mortality in the multivariate model.”
In the discussion section, Moat and colleagues further described that the “high attrition in the first year post-implant is also seen in the SOURCE registry and the Italian registries and in both cohorts of the PARTNER trial; for example, 18% of patients died after a TAVI between 30 days and 1 year in PARTNER A. It is of interest that there was an almost identical rate of attrition in the control (AVR) group.” The authors additionally noted that the incidence of early stroke was comparable to other registries and to the PARTNER trial, as well as that “the finding of magnetic resonance imaging evidence of (albeit seemingly silent) cerebral perfusion defects in 84% of TAVI patients highlights the need to evaluate neurological outcomes in these patients, including cognitive function. Embolic protection devices may have a role in ameliorating the incidence of stroke, but at present it remains a major concern and represents an obstacle to the application of TAVI in lower risk patients.”
Moreover, “in 61% of patients, there was a degree of paravalvular AR [aortic regurgitation] that would traditionally have been regarded as suboptimal or even unacceptable after AVR. The finding that the degree of post-implant AR was an independent predictor of survival at 1 year is an important observation and requires further detailed study. Whether the regurgitation is responsible for this adverse outcome or is merely a marker for other adverse features cannot be assessed from this registry. The presence of moderate or severe AR was more common in the Medtronic CoreValve cohort. There is some evidence that the degree of AR remains stable or even reduces during the first year post-implant. The influence of this residual AR on parameters such as the incidence of endocarditis and hemolysis and the effect on LV mass regression are unknown and will need to be further evaluated. A reduction in the incidence and severity of paravalvular AR represents an obvious target for technical improvements in the design of transcatheter valves and of implantation techniques.”
The authors also acknowledged that “the observation that COPD was an independent predictor of outcome is perhaps surprising. In patients with aortic stenosis and COPD, it can be difficult to be certain as to the precise contribution of each pathology in an individual patient with progressive severe breathlessness. For patients in whom COPD predominates [COPD was significantly greater in controls compared to TAVI patients in the PARTNER trial’s Cohort B], the relief of aortic stenosis may not change the clinical outcome as much as in other patient groups, and that may in part explain this observation.”
In conclusion, Moat and colleagues stated that “Midterm to long-term survival after TAVI was encouraging in this high-risk patient population, although a substantial proportion of patients died within the first year.”
Complications
Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 2011 June;97(11):899-906. PMID: 1398694
Abdel-Wahab and colleagues concluded that “significant AR [aortic regurgitation] after TAVI is common and is associated with increased in-hospital mortality. Long-term follow-up is critical to further define the impact of residual AR on clinical outcome. Until these data become available, every effort should be made to prevent and treat this complication.”
Frailty
Makary M, Segev D, Pronovost P, et al. Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons 2010 June;210(6):901-908.
Makary and colleagues from the John Hopkins University School of Medicine in Baltimore concluded: “Frailty independently predicts postoperative complications, length of stay, and discharge to a skilled or assisted-living facility in older surgical patients and enhances conventional risk models. Assessing frailty using a standardized definition can help patients and physicians make more informed decisions.”
Afilalo J. Frailty in patients with cardiovascular disease: why, when, and how to measure. Current Cardiovascular Risk Reports 2011 October;5(5):467-472.
Afilalo from McGill University in Montreal concluded: “Frailty and CVD [cardiovascular disease] share common biological pathways, and CVD may accelerate the development of frailty. Frailty is identified in 25% to 50% of patients with CVD, depending on the frailty scale used and the population studied. Frail patients with CVD, especially those undergoing invasive procedures or suffering from coronary artery disease and heart failure, are more likely to suffer adverse outcomes compared to their non-frail counterparts. The 5-m gait speed test is a simple and effective way of objectively measuring frailty in patients with CVD and should be incorporated in risk assessment. Further research will clarify how to best incorporate frailty in existing risk models and how to optimize health status and prevent adverse outcomes in frail patients.”
Zenilman M, Chow W, Ko C, et al. New developments in geriatric surgery. Current Problems in Surgery 2011 October;48(10):670-754.
Zenilman and colleagues from Johns Hopkins Medicine in Baltimore reported that: “Frailty as a marker of a patient’s ability to tolerate stress has been validated by its ability to predict complications following surgery – the greatest stress test a person can withstand,” as well as that “The prevalence of frailty among those over age 65 has been estimated as between 7% and 16% and is more common among women and African American individuals. Among those presenting for elective surgery over age 65, the prevalence has been estimated at 11% being frail, and 41% being at least intermediately frail.”
4. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
CMS did not hold a MEDCAC meeting on this topic.
5. Evidence-Based Clinical Guidelines
There currently are no evidence-based guidelines for this procedure.
6. Professional Society Position Statements
On January 10, 2012, the American College of Cardiology (ACC) submitted a letter to CMS accompanied by a preliminary guidance on Institutional and Operator Requirements for TAVR supported by multiple specialty medical societies – ACC, American Association for Thoracic Surgery (AATS), Society for Cardiovascular Angiography and Interventions (SCAI) and the Society of Thoracic Surgeons (STS). The letter, available in Appendix C, states the following:
Please understand that the attached should be viewed as preliminary guidance on institutional and individual operator qualifications for performing TAVR, and we want to emphasize that this interim information may change in the official and forthcoming document. Given the urgency for providing information, the attached represents the current best thinking of the four societies’ leaders in these regards, as has been conveyed to the societies from the scientific writing committee as they are finalizing the official document.
The preliminary guidance states the following:
- For New Centers to be considered for TAVR:
- Institutional Requirements:
-Surgical:
- ≥ 50 total AVR procedures/year, with ≥ 10 High Risk (STS Score ≥ 6)
- ≥ 2 institutionally-based cardiac surgeons
- Interventional:
- ≥ 400 caths/150 PCIs per year
- ≥ 15 left-sided structural (EVAR,TEVAR, etc.) interventions
- Individual Requirements:
Surgeon:
- Board Certified/Eligible in CV Surgery.
- Professional experience with:
- ≥ 100 AVR/career including 10 high-risk patients OR
- ≥ 25 AVR/Year or 50 AVR in 2 years AND
- ≥ 20 in the last year prior to TAVR
Interventionalist:
- Board Certified/Eligible in Interventional Cardiology
- Professional experience with:
- 50 structural heart disease procedures
- Existing TAVR Centers:
- Participation in ongoing TAVR programs, either PAS or RCTs
- Experience with ≥ 30 TAVR procedures and ≥ to 20 /year
TAVR Program
- ≥ 20 procedures/year OR 40 procedures/2 years
- 30 day all-cause mortality ≤ 15%
- 30 day neurologic events ≤ 15%
- 30 day major vascular complications ≤ 15%
- ≥ 90% institutional follow-up
- ≥ 60% one-year survival for non-operable patients
- For all centers either new or established:
- Participation in National TAVR Registry for ongoing enrollment and follow up of all TAVR patients
- Commitment to Heart Team concept
- Adherence to the ACCF/AATS/SCAI/STS expert consensus document on TAVR
7. Public Comments
During the initial 30-day public comment period (09/28/2011 - 10/28/2011), CMS received 30 public comments. All comments were generally supportive of coverage for TAVR, however some commenters strongly suggested that coverage be left to local Medicare contractors and not be handled through the NCD process. One commenter supported coverage in clinical trials. Several commenters cited concerns regarding access due to aspects of the formal request surrounding center of excellence requirements, facility requirements, staffing requirements and volume thresholds. Several commenters offered suggestions on how the formal request could be revised to address some of these concerns.
CMS received seven comments from professional societies and education and advocacy associations; seven from individuals who did not identify an associated organization or profession; six from medical facilities, physicians and researchers; four from hospital administrators; three from lawmakers; and three from device manufacturers.
The comments can be viewed in their entirety on our website at https://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=257&ExpandComments=n&ver=3&NcaName=Transcatheter+Aortic+Valve+Replacement+(TAVR)&bc=ACAAAAAAIAAA&.
VIII. CMS Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1862(l) of the Act).
In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, section 1862(a)(1) of the Social Security Act in part states, with limited exceptions, no payment may be made under part A or part B for any expenses incurred for items or services:
- Which, are not reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member (§1862(a)(1)(A)) or
- in the case of research conducted pursuant to section 1142, which is not reasonable and necessary to carry out the purposes of that section. ((§1862(a)(1)(E)).
Section 1142 of the Social Security Act describes the authority of the AHRQ. Under section 1142, research may be conducted and supported on the outcomes, effectiveness, and appropriateness of health care services and procedures to identify the manner in which diseases, disorders, and other health conditions can be prevented, diagnosed, treated, and managed clinically
Section 1862(a)(1)(E) allows Medicare to cover under coverage with evidence development (CED) certain items or services for which the evidence is not adequate to support coverage under section 1862(a)(1)(A) and where additional data gathered in the context of a clinical setting would further clarify the impact of these items and services on the health of Medicare beneficiaries. For your convenience, the 2006 CED guidance document is available at http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/ced.pdf.
As noted earlier, our review sought answers to the questions below. We have repeated them here for the convenience of the reader.
- Is the evidence adequate to conclude that transcatheter aortic valve replacement improves health outcomes for Medicare beneficiaries with severe symptomatic aortic stenosis who are not candidates for surgical aortic valve replacement? [PARTNER Cohort B]
- Is the evidence adequate to conclude that transcatheter aortic valve replacement improves health outcomes for high surgical risk Medicare beneficiaries with severe symptomatic aortic stenosis who are candidates for surgical aortic valve replacement? [PARTNER Cohort A]
If the answer to either or both of the questions above is positive, is the available evidence adequate to identify the characteristics of the patient, practitioner or facility that predict which beneficiaries are more likely to experience overall benefit or harm from TAVR?
TAVR first-in-man was performed in 2002, and TAVR received Europe’s CE mark in 2007. Since then, it has been performed in patients at risk of complications from the surgical procedure though (considering the subjective nature of risk scoring) it is difficult to ascertain how many were “high-risk” as defined by the pivotal PARTNER trial. Prior to the PARTNER randomized trial, published evidence consisted of cases series and non-randomized comparative studies; and assessing mortality and morbidity in these studies is difficult due to patient selection bias, lack of standardized definitions for endpoints, variable center and operator experience, plus incomplete patient follow-up. “It is difficult to compare outcomes in non-randomised comparative studies since patients who are selected for TAVI are likely to be more ill and more likely to suffer complications or die” (NICE 2011).
The PARTNER Cohort A and Cohort B trials were randomized controlled trials designed for FDA pre-market approval and were designed to address indications, efficacy and patient safety issues. Of over 3,000 patients screened, 34% underwent randomization and were divided into two cohorts, all being very highly selected high-risk patients with severe symptomatic native aortic stenosis (AS). Partner A patients were those who were considered by at least two surgeons to be suitable for surgery (despite the fact that they were at high risk), whereas Cohort B patients were not considered to be suitable for surgery in the opinion of at least two surgeons. From that point, they were randomized (in Cohort A) to either surgical or transcatheter (either transfemoral or transapical) aortic valve replacement, and (in Cohort B) to either transcatheter (transfemoral) valve replacement or the standard therapy control group (78 % of whom received balloon aortic valvuloplasty).
Viewing baseline characteristics as a reflection of randomization, while Cohort A was balanced, there was a definite imbalance in Cohort B (COPD, atrial fibrillation, logistic EuroSCORE). A superiority design was chosen for the inoperable Cohort B patients, with TAVR compared to standard therapy; and as noted above, most patients in the standard therapy group received balloon aortic valvuloplasty – a procedure which is not considered to be overly efficacious but rather a bridge to open or transcatheter treatment or palliation. However, for the operable high-risk Cohort A patients (Smith 2011), a non-inferiority study design was chosen – the rationale for which, instead of superiority testing as in Cohort B, is not straightforward (Neyt 2011) and can now be questioned since net harms have outweighed benefits for TAVR patients in Cohort A.
Though all patients in Cohort B were deemed inoperable, twelve patients had conventional open AVR. Historically, surgical risk was a decision based on the personal experience of the surgeon. There are a number of risk scoring systems that have been developed, but their usefulness is variable. Although many morbidity and mortality risk factors for these scores have been extensively analyzed, there continues to be uncertainty about who will experience adverse outcomes. This is especially true in the elderly (Saxton and Velanovich 2011). Attempts have been made to provide objective risk criteria, but considerable subjectivity of when and who should be operated on remains. Ultimately, any procedure is a risk-benefit decision, and it is important to accurately estimate this information for patient decision-making.
In Cohort B, the primary outcome was rate of death at one year from any cause; and TAVR did demonstrate superiority in the PMA trial. Also, the co-primary endpoint (a composite of death and recurrent hospitalization) was 42.5% with TAVI and 71.6% with standard therapy; but this composite endpoint was only added later and was not one of the trial’s original endpoints (FDA Executive Summary 2011). Among those who did survive to one year, the rate of cardiac symptoms (as judged by NYHA class) was lower among patients who had undergone TAVI than among those who received standard therapy. But optimal management of coronary artery disease (CAD) in the setting of TAVI is not well defined, since patients with recent or untreated clinically significant CAD requiring revascularization (as well as significant peripheral vascular disease) were systematically excluded from the PARTNER trial.
Critically, however, while one year mortality was an absolute 20% lower for patients who underwent TAVI as compared to standard therapy, the Belgian technology assessment (Neyt 2011) analyzed and noted – as was discussed in considerable detail at the FDA panel meeting on July 20, 2011 – that one year mortality was in fact an absolute 12.7% higher for 90 patients who underwent TAVI compared to standard therapy in the PARTNER trial’s continued access study (a continuation of the PARTNER RCT).
Of 179 patients assigned to TAVI, six did not receive a valve; several received more than one valve; and four underwent valve-in-valve procedures. Regarding these valve-in-valve cases, the FDA Executive Summary [page 29] cautioned that if TAVI “becomes commercially available, widespread use of the valve-in-valve technique might occur. While this only occurred four times in the Cohort B study, there have been many reports of valve-in-valve usage in Europe. Without any pre-clinical testing, and limited clinical data, the FDA is unable to draw conclusions regarding the short- and long-term safety of SAPIEN valve-in-valve implantation.” CMS shares these concerns.
Adverse events were not equal between the inoperable Cohort B groups. Strokes, vascular events, and major bleeding were higher in the TAVI group than the standard therapy group. Stroke occurred more frequently in the TAVI group than in the standard therapy group at both 30 days (6.7% versus 1.7%, p = 0.03) and at one year (10.6% versus 4.5%, p = 0.04). However, in Table 6 of the FDA Executive Summary (updated June 11, 2011), the number of strokes observed in the TAVI group and reported at the panel meeting were higher than those published by Leon, et al. (2010), i.e., 7.3% at 30 days and 11.2% at one year.
Furthermore, in the ITT (intention-to-treat) population, the number of all neurological events (stroke and TIA) over the entire study period was more than three times higher in the TAVI group (N = 25, 14%) as compared to the control group (N = 8, 4.5%). A more detailed examination reveals that the eight neurological events observed in standard therapy controls over the entire study period included no TIAs. Rather, in control patients, there was one hemorrhagic stroke at eight months and seven ischemic/ unclassified strokes: one after open AVR; four after BAV (five days, two weeks, two months, six months); two in patients who only received medical management (one on the day of randomization, and another three days after randomization) (FDA Executive Summary).
The FDA executive summary also noted: “Only 14 control patients had optimal medical therapy without an interventional procedure throughout the trial. As mentioned above, two of these 14 patients had strokes within 3 days of randomization, but there were no further strokes. Fourteen additional patients had either open AVR or apico-aortic conduits. One of these 14 patients had a stroke on the day of surgery. There were no further strokes throughout the trial in the Control group. Therefore, the control group had minimal neurological events over 60 days after invasive procedures and there does not appear to be a continuing risk of neurological events. As a result, there is no evidence that the patients in this study were a high risk stroke population.”
Moreover, in Cohort B’s TAVI group, there were three TIAs (143 days in one patient; 386 and 831 days in a second patient), three intracranial hemorrhages (51, 136, 151 days), three hemorrhagic strokes (two, 39, and 120 days), and 16 ischemic/unclassified strokes: one occurred after randomization and before device implantation; 10 of 16 were recognized within six days of implantation or attempted implantation; two of 16 occurred from 23-180 days (23, 75 days); and three of 16 occurred late (361, 650, 875 days). “This shows that 12/25 (48%) of the neurological events occurred > 30 days after the procedure – thus indicating a continued risk of neurological events with the device” (FDA Executive Summary 2011, page 23).
TAVI was also associated with a higher incidence of major vascular complications at 30 days (16.2% versus 1.1%, p < 0.001), as compared with standard therapy; and in the summary prepared for the July 20, 2011 Circulatory System Devices Panel meeting, the FDA reported that half (55.9%) of the TAVI patients had serious adverse events relating to the access procedure. Based on their review of the Clinical Events Committee narratives, the FDA Executive Summary (page 24) noted that the most serious of these vascular complications included: aortic dissection (1 patient), iliac artery/distal aortic injury (17 patients), femoral artery injury (13 patients), pseudoaneurysm (2 patients), hematoma (6 patients) and unknown injury (2 patients). The TAVI patients do receive heparin during the procedure and are anticoagulated post-procedure, but it is not clear if bleeding events are attributable to this anticoagulation.
Several groups (Kahlert 2010, Ghanem 2010, Arnold 2010, Rodes-Cabau 2011, Astarci 2011) have also published series documenting the rate of clinically silent cerebral ischemia following TAVI. Kahlert and colleagues (2010), for instance, assessed peri-procedural apparent and silent cerebral ischemia by neurological testing and serial cerebral diffusion-weighted magnetic resonance imaging (DW-MRI) at baseline, 2.5 to 4.4 days after the procedure, and at three months after TAVI. Clinically silent new foci were detected in almost all patients (84%) undergoing TAVI in Kahlert’s series, which (while typically multiple) were not associated with apparent neurological events or measurable deterioration of neurocognitive function during three-month follow-up. The clinical significance of these findings is unclear, with further investigative work needing to be done to inform patient understanding of the procedure.
A paravalvular leak refers to aortic regurgitation (AR) occurring due to mismatching of the implanted prosthetic valve and native aortic annulus, such that during diastole, part of the forward blood flow into the ascending aorta flows back between the prosthesis and the annulus. In Cohort B, moderate or severe paravalvular aortic regurgitation (AR) was present in 11.8% of TAVI patients at 30 days and in 10.5% of TAVI patients at one year. Additionally, when both central regurgitation and paravalvular leak were included,15.6% of TAVI patients had moderate or severe aortic regurgitation at one year. The FDA Executive Summary (page 25) noted that this amount of AR was appreciable and did not decrease over time in the TAVI group, and that the degree of AR remains a concern which will need to be monitored in subsequent studies. Critically, the presence of moderate to severe AR has been recently shown to be an independent predictor of mortality (Moat 2011; Abdel-Wahab 2011).
While outcomes for Cohort A have been reported (Smith 2011), at the time of this national coverage analysis the data for this cohort remains under FDA review; and no panel meeting has yet been scheduled. Here also the primary endpoint is death from any cause at one year in the intention-to-treat population, and there was a nonsignificant difference that was within the trial’s non-inferiority margin (24.2% in the TAVR group versus 26.8% in the surgical AVR group) for PARTNER Cohort A. Notably, however, greater than 10% (N = 38) of the patients randomized to surgical replacement in Cohort A were not treated as assigned – mainly due to patient refusal or withdrawal, including 27/248 surgical replacement control patients in the transfemoral cohort and 11/103 surgical replacement control patients in the transapical cohort (N = 103). Only 4/244 (1.1%) patients in the transfemoral TAVR group were not treated as assigned by randomization. Recurrent hospitalization rates was a secondary endpoint in Cohort A, and at one year rehospitalization occurred in 58 patients (18.2%) in the TAVR group and in 45 (15.5%) in the surgical AVR group, a non-statistically significant difference. The functional outcomes of NYHA class and 6-minute walk showed a difference at 30 days, but at one year the earlier between-group differences were not evident. The one year QOL results for PARTNER’s Cohort A (while publicly presented) have yet to be published.
In Cohort A, adverse events were not equal between the TAVI group and the surgical AVR group. Strokes and vascular events were higher in the TAVI group than the surgical group, with major bleeding higher in the surgical group. Stroke was a pre-specified secondary end point, and all neurological events (comprising major stroke, minor stroke and TIA) occurred more frequently in the TAVR group than in the surgical AVR group, both at 30 days (5.5% versus 2.4%, p = 0.04) and at one year (8.3% versus 4.3%, p = 0.04). Major strokes (while not a pre-specified endpoint) likewise occurred more frequently in the TAVR group than in the surgical AVR group, both at 30 days (3.8% versus 2.1%, p = 0.20) and at one year (5.1% versus 2.4%, p = 0.07). Vascular complications and bleeding were additional pre-specified secondary safety endpoints. At 30 days, the TAVR group had a significantly higher rate of vascular complications than did the surgical AVR group (17.0% versus 3.8%, p < 0.001); and the TAVR group had significantly higher rate of major vascular complications[1] than did the surgical group (11.0% versus 3.2%, p < 0.001).
Notably, in PARTNER Cohort A, moderate or severe paravalvular AR occurred more frequently in the TAVR group versus the surgical AVR group at both 30 days (12.2% versus 0.9%, p < 0.001) and at one year (6.8% versus 1.9%, p < 0.001). In Moat and colleagues’ (2011) all-inclusive TAVR registry series, follow-up ranged from 11-46 months; and mortality tracking was achieved in 100% of patients with survival status reported as of December 12, 2010. In data collected prospectively for Moat’s consecutive series of 870 high-risk patients undergoing 877 TAVR procedures (regardless of technology or access route) in all 25 centers undertaking TAVR in the United Kingdom, the presence of moderate to severe AR was an independent predictor of mortality. Likewise, in another recent large series evaluating post-procedural AR in 690 of 697 consecutive transcatheter aortic valve implantations, Abdel-Wahab (2011) found that significant (≥ 2/4) AR occurred in 119 patients (17.2%) and was a strong independent predictor of in-hospital death.
Finally, the spectrum of aortic stenosis is a continuum and some patients with severe symptomatic aortic stenosis are either too sick or have such severe comorbidities that, despite TAVI, these patients – the so-called “Cohort C” patients described in the FDA Executive Summary (page 29) – will not improve functionally or live longer following intervention:
“The FDA worked extensively with sponsor to define “inoperable” or “extreme high risk” for this randomized study of inoperable patients so as not to enroll less sick patients who could reasonably have open AVR. However, active consideration was not given to specifying patients who should not have transcatheter valve implantation due to extensive comorbidities. There were no specific inclusion/exclusion criteria in this study to eliminate patients too sick to benefit from isolated treatment of severe aortic stenosis.
Based on a review of the CEC narratives, it is clear that one needs also to consider when transcatheter valve implantation may not have a positive impact on a patient’s quality of life. In addition, SAPIEN implantation requires general anesthesia, 4+ hours of procedure time, radiographic contrast, invasive TEE, often an operative procedure for vascular access or closure, etc.; therefore, it is a highly invasive interventional cardiology procedure.”
Likewise of importance, more than death itself, many elderly patients fear loss of independence, becoming a burden to family, and/or nursing home admission. For such individuals considering surgical or transcatheter AVR, stroke may be worse than death.
- Is the evidence adequate to conclude that transcatheter aortic valve replacement improves health outcomes for Medicare beneficiaries with severe symptomatic aortic stenosis who are not candidates for surgical aortic valve replacement? [PARTNER Cohort B]
For the highly selected patients in Cohort B, the evidence is not adequate to conclude that TAVR generally improves health outcomes for Medicare beneficiaries with severe symptomatic native aortic stenosis who were deemed not to be suitable candidates for surgical AVR. TAVR, however, may improve health outcomes in very highly selected, well-informed, inoperable patients when added safety and patient protections are in place in carefully monitored clinical studies performed by expert multi-disciplinary heart teams in facilities that furnish an appropriate environment, which can be available through CED under §1862(a)(1)(E). We believe that the STS ACC TAVT Registry, as currently designed, is an appropriate platform for a carefully monitored clinical study for this purpose. We also believe that adherence to facility and practitioner criteria based on guidance developed jointly by AATS, ACCF, SCAI and STS should be considered to satisfy the CMS requirements for facilities and practitioners.
- Is the evidence adequate to conclude that transcatheter aortic valve replacement improves health outcomes for high surgical risk Medicare beneficiaries with severe symptomatic aortic stenosis who are candidates for surgical aortic valve replacement? [PARTNER Cohort A]
For the highly selected patients in Cohort A with severe symptomatic native aortic stenosis who were deemed candidates for surgical aortic valve replacement, TAVR provided no mortality benefit but significant risk of harms. TAVR, however, may yet be demonstrated to improve health outcomes in very highly selected, well-informed, operable patients seeking added safety and patient protection available in carefully monitored clinical studies performed by expert multi-disciplinary heart teams in facilities that furnish an appropriate environment under §1862(a)(1)(E). While this remains an unlabeled use, we believe that Medicare coverage for this patient population should be restricted only to clinical trials rather than registries.
We believe that gaps in the current evidence base lead to uncertainty about the overall impact of TAVR on beneficiary outcomes when furnished outside of the setting of clinical trial protocols. The following points describe some of our concerns.
- The STS risk score and EuroSCORE give operative risk information, but do not predict the important patient-centered outcome of quality of life improvement. For patient selection and informed consent, information about quality of life improvement as it applies to individual patient decision making should be available.
- Accurate risk prediction is important. There are no specific recommendations for defining inoperability so this depends on the judgment of the medical team. Assessment can vary and be dependent on surgeon and institutional experience. A clearer understanding of comorbid conditions that affect patient outcomes is crucial. Furthermore, the impact of “unmeasured covariates” that enter into “clinical judgment” is unknown and likely critical for patient outcomes (Sundt 2009). Better tools are needed to assist both physicians and patients in risk ascertainment.
- Mortality (all-cause) at 30 days was less in the randomized PARTNER trial data as compared to the SOURCE registry (consecutive patients in Europe after commercialization) (Thomas 2010). Therefore, it remains unclear if the randomized trial data that were generated under optimal procedural circumstances is generalizable to routine clinical practice.
- The clinical significance of clinically silent cerebral ischemia is unknown. An examination of both short and long term quality of life information is needed to inform patient understanding of this procedure.
- The assessment of treatment success should encompass the reasonably expected durability of the treatment and extend beyond the mere technical completion of the operative procedure.
- Mixed results in the evidence base to date may reflect differences that may be predictable. Utilizing the CED approach is important to ensure that future care is informed by lessons learned.
The learning curve with this complex technology appears substantial (Nuis 2011, Alli 2011). For instance, Gurvitch 2011 suggests that procedural experience is an independent predictor of 30-day mortality. In a recent study by Alli, they stated, “Our data show increasing proficiency with evidence of plateau after the first 30 cases. More studies are needed to confirm these findings”(Alli 2011). In this cohort at the Mayo clinic, the 30-day mortality was 11%, clearly higher than the PARTNER results. The correlation of volume to mortality and morbidity can be clarified with additional evidence.
The data used for the FDA PMA approval were generated under rigorous clinical trial conditions. To enhance the likelihood that beneficiaries experience similar improved health outcomes overall, operator and facility criteria are important and need definition. The formal request for this analysis broadly outlined desirable operator and facility criteria for performing TAVR. Our criteria, listed in section I of this document, are informed by the information included in the formal request and subsequent information submitted to CMS by the requestors such as the preliminary operator and facility guidance sent to CMS on January 10, 2012 (available above in section VII B 6 and Appendix C of this document) as well as our own review of the evidence. As such, we are proposing the operator and facility criteria that we believe are appropriate in section I of this document.
We understand that the professional societies have released a manuscript accepted for publication in various journals including the Journal of the American College of Cardiology (JACC) entitled 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement (Holmes 2012). The article may be found online at http://content.onlinejacc.org/cgi/reprint/j.jacc.2012.01.001v1.pdf. This document was released immediately prior to the release of this proposed decision memorandum. We anticipate a full review and incorporation of this document into our final decision memorandum.
In this rapidly evolving technology, the incidence of stroke and other adverse outcomes may decline with improvements in patient selection, device characteristics, and procedural practices. Monitoring these changes that ultimately lead to improvements in morbidity and mortality is critical.
Disparities in Transcatheter Aortic Valve Replacement
In the PARTNER Cohort B trial, 46% of study participants were men and 92% were Caucasian. Makary (2010) reported that frailty independently predicts postoperative complications, length of hospital stay, and discharge to a skilled or assisted-living facility in older surgical patients, and Zenilman (2011) noted that prevalence of frailty among those > 65 years old has been estimated at 7-16% and is more common among women and African Americans.
Summary
Upon review of the available evidence, we believe that the requestors’ arguments are generally persuasive and we believe that our proposed decision to cover labeled uses using the Coverage with Study Participation form of CED is consistent with the request from the professional societies. The requestors presented reasonable and supportable arguments for restricting coverage for labeled uses to practitioners and facilities meeting specified criteria, the derivation of which is from a consensus (Holmes 2012) among the professional societies, and for requiring ongoing data collection. We agree that robust and reasonable practitioner and facility criteria can be articulated and that their presence will improve beneficiary outcomes. We believe that CMS criteria for data collection and analysis for FDA approved labeled indications can be met through enrollment and participation in a national prospective TAVR registry. We also believe that this proposed decision is consistent with the FDA requirement for continuing data collection and analysis.
We believe that there is promising but inadequate evidence to conclude at this time that TAVR generally improves health outcomes for Medicare beneficiaries with severe symptomatic aortic stenosis. We believe that the beneficiaries’ ability to attain improved health outcomes is maximized when TAVR is furnished in settings that reflect those in the pivotal PARTNER trial, by appropriately trained, experienced operators in the context of a multidisciplinary team in a setting that assures sufficient volume to maintain proficiency. We are also mindful of ongoing research and recognize that an alternative to open surgical aortic valve replacement may be clinically appropriate and preferable in selected patients when certain protections are in place to enhance the likelihood of benefit. We also believe that the additional data collected in the context of a setting can further clarify the impact of TAVR on the health of this Medicare patient population. We believe that Medicare coverage under the Coverage with Study Participation CED paradigm balances these considerations in the interests of our beneficiaries.
It is not apparent to us at this time that the available evidence clearly distinguishes patients who will experience an improved outcome from those who will derive harm such as a stroke or death, especially beyond one year post TAVR. Given the availability of an effective treatment - open surgical valve replacement – we believe that additional evidence needs to be developed to better inform treatment decisions and for fully informed discussions of risk and benefit of TAVR in operable patients with severe symptomatic aortic stenosis. We propose that this evidence can be developed in the context of clinical studies that meet the criteria specified in section B of the proposed decision.
There are inherent challenges in developing durable conclusions about an invasive technically complex surgical procedure when much of the non-trial experience has accrued in other countries. Though technical factors and underlying patient physiology would be expected to vary little among countries, the practice of medicine reasonably reflects cultural expectations and local incentives for the behaviors of patients and physicians that may not align with those factors in the United States.
The success of surgical procedures depends heavily on the skill and experience of the operator(s) and the supporting environment for the procedure itself and for postoperative care of the patient. We recognize, as the requestors have noted, that new technologies demonstrate a learning curve. This leads logically to caution about expecting that results achieved by selected experts working within the parameters of a formal clinical trial protocol will be seen when the technology is disseminated to less experienced operators in non trial settings. Experience also tells us that, with disseminated use over time, adverse event signals may become stronger and more apparent than initial data have indicated. At the same time, we believe that reported health outcomes can improve over time as operators gain more training and experience and as the collective experience leads to improvements in the technology itself. Both of these are relevant to TAVR.
For patients with severe symptomatic native aortic stenosis in Smith and colleagues’ (2011) PARTNER Cohort A who were deemed to be high surgical risk for surgical AVR, transcatheter aortic valve replacement provided no mortality benefit but rather increased risk of harms, including both significantly increased rates of stroke at 30 days and one year, as well as significantly increased vascular complications at 30 days.
That is, while TAVR may at some time in the future reduce overall morbidity and mortality for a better defined subset of high surgical risk patients, such a result has not yet been conclusively demonstrated. Furthermore, adopting this potentially transformational technology for use in moderate or lower risk populations beyond the selected high surgical risk population studied in the PARTNER trial is not appropriate at the current time.
Furthermore, where frailty was assessed by quantifying ability of patients to perform activities of daily living, as well as by performing a hand grip and a walk test, frailty was more often present in the standard therapy controls than TAVI patients (28.0% versus 18%) in PARTNER Cohort B. Such imbalance in both frailty as a baseline characteristic, as well as the overwhelming lack of racial diversity in the study participants who were enrolled, severely limits both the internal and external validity of the PARTNER Cohort B trial.
We have noted the absolute 12.7% increased mortality for TAVI as compared to standard therapy reported for the 90 randomized patients in PARTNER’s Continued Access study. Specifically, for patients with severe symptomatic native aortic stenosis in Leon and colleagues’ (2010) PARTNER Cohort B who were jointly deemed inoperable by a cardiologist and at least two cardiovascular surgeons and who then underwent transcatheter aortic valve implantation, the pivotal trial’s published mortality benefit of 20% – while promising for some patients who may not fear stroke more than death – is not generalizable beyond this very highly selected study population and may overestimate TAVI’s treatment effect – particularly when one considers the uneven distribution of baseline characteristics (especially atrial fibrillation, COPD and frailty) which were greater in the standard therapy controls. Moreover, in PARTNER Cohort B, strokes were significantly more frequent in the TAVI group at both 30 days and at one year as compared with standard care; and TAVI was associated with significantly greater major vascular complications at 30 days as compared with standard therapy.
Critically, in addition to the notably high incidence of incompletely understood, clinically silent cerebral emboli detected by DW-MRI following TAVI, patients in Cohort B who underwent TAVI experienced two and a half times more strokes than those who did not receive an implanted valve; and nearly half (48%) of the neurological events (stroke and TIA) in Cohort B occurred more than 30 days after the procedure – which as noted by the FDA indicated a “continued risk of neurological events with the device.”
Overarching concerns for TAVI are therefore high incidence of paravalvular aortic regurgitation (rare in surgical AVR) and post-procedural strokes that are possibly related to embolic material from the device itself and/or the unopposed space (paravalvular leak) between the implanted valvular prosthesis and the native aortic annulus. Such serious sequelae would be magnified in younger patients and/or lower to moderate risk patient populations with fewer comorbidities and longer life expectancy.
Results of the PARTNER trial cannot be extrapolated beyond the very highly selected Cohort A and Cohort B study populations or beyond the expert multidisciplinary heart teams and specialized facilities utilized in the PARTNER trial. Therefore, we believe that Medicare beneficiaries are more likely to experience the best achievable outcomes when TAVR is furnished in a manner that replicates the safeguards contained in the PARTNER protocol. We recognize the distinction between protocol requirements that address the clinical delivery of the then-investigational item or service itself from those that address administrative aspects of clinical research. We believe this proposed decision reflects an appropriately balanced consideration of the topic, mindful of beneficiary outcomes and clinical efficiency.
IX. Conclusion
CMS proposes that the available evidence is insufficient to conclude that transcatheter aortic valve replacement (TAVR) improves beneficiary health outcomes in Medicare beneficiaries for the treatment of severe symptomatic aortic valve stenosis. We therefore propose that coverage for TAVR for this indication is not reasonable and necessary under section 1862(a)(1)(A) of the Social Security Act . However, we believe that TAVR may, upon the development of additional evidence, prove to represent a substantial benefit to Medicare beneficiaries with severe symptomatic aortic valve stenosis, especially those for whom open surgical aortic valve replacement would be contraindicated or high risk. Therefore, CMS proposes that coverage for TAVR be approved under section 1862(a)(1)(E) of the Act, Coverage with Evidence Development (CED), only for the following conditions and as specified below:
- TAVR is covered for the treatment of severe symptomatic aortic valve stenosis only, when all of the following conditions 1-5 are met.
- The procedure is furnished for an FDA approved indication, with a complete valve and implantation system that has received FDA premarket approval (PMA) for this indication.
- Two cardiac surgeons have, according to the pivotal PMA trial’s protocol, evaluated the patient’s suitability for open valve replacement surgery.
- The procedure is furnished in a facility that meets the following institutional requirements:
- For centers without previous PMA clinical trial TAVR experience
- Surgical program requirements:
- ≥ 50 total aortic valve replacement (AVR) procedures/year, including ≥ 10 patients with STS (Society of Thoracic Surgeons) Score ≥ 6;
- ≥ 2 institutionally based cardiac surgeons.
- Interventional program requirements:
- ≥ 400 caths/150 PCI’s (percutaneous interventions) per year;
- ≥ 15 left-sided structural (EVAR [endovascular aneurysm repair], TEVAR [thoracic endovascular aortic repair], etc.) interventions per year.
- For centers with previous PMA clinical trial TAVR experience
- Participation in ongoing TAVR programs, either randomized controlled trials (RCTs) or post-approval study (PAS);
- Experience with ≥ 30 TAVR procedures and ≥ 20/year;
- TAVR program requirements:
- ≥ 20 procedures/year OR ≥ 40 procedures/2 years;
- 30 day all-cause mortality ≤ 15%;
- 30 day neurologic events ≤ 15%;
- ≥ 90% institutional follow-up of patients;
- ≥ 60% one year survival for non-operable patients.
- For all centers, with or without previous PMA clinical trial TAVR experience:
- Participation in a prospective national TAVR study for ongoing enrollment and follow up of all TAVR patients;
- Commitment to Heart Team concept.
- The procedure is performed by physicians with the following qualifications and experience:
- Surgeon requirements:
- Board Certified/Eligible in Cardiovascular Surgery;
- Professional experience with:
- ≥ 100 AVR/career including 10 high risk patients; OR
- ≥ 25 AVR/year or 50 AVR in 2 years; AND
- ≥ 20 in the last year prior to TAVR.
- Interventionalist requirements:
- Operators must be Board Certified/Eligible in Interventional Cardiology
- Professional experience with 50 structural heart disease procedures
- The patient is enrolled in, and the treating physician team is participating in a prospective national registry that consecutively enrolls TAVR patients and tracks at least the following outcomes at the patient data level for a period of at least five years from the time of the TAVR procedure.
- Major stroke;
- All cause mortality;
- Minor stroke/TIA;
- Major vascular events;
- Acute kidney injury;
- Repeat aortic valve procedures;/li>
- Quality of Life measures.
The registry must be designed to permit identification and analysis of patient, practitioner and facility level factors that predict patient risk for these outcomes. The patient must have, after being informed of the reported risks of TAVR and reasonable alternative management strategies, given informed consent.
- Except as specified under A. above or C. below, CMS proposes coverage for all unlabeled uses of TAVR only when all of the following conditions are met:
- TAVR is covered in clinical studies that fulfill criteria a-m below and have characteristics i-ii.
- Superiority (not non-inferiority) TAVR study design; and
- Where TAVR is performed by a multi-disciplinary heart team that includes cardiologist(s) and cardiac surgeon(s) jointly participating in intra-operative technical aspects of TAVR.
The clinical study must adhere to the following standards of scientific integrity and relevance to the
Medicare population:
- The principal purpose of the research study is to test whether a particular intervention potentially improves the participants’ health outcomes.
- The research study is well supported by available scientific and medical information or it is intended to clarify or establish the health outcomes of interventions already in common clinical use.
- The research study does not unjustifiably duplicate existing studies.
- The research study design is appropriate to answer the research question being asked in the study.
- The research study is sponsored by an organization or individual capable of executing the proposed study successfully.
- The research study is in compliance with all applicable Federal regulations concerning the protection of human subjects found in the Code of Federal Regulations (CFR) at 45 CFR Part 46. If a study is regulated by the Food and Drug Administration (FDA), it also must be in compliance with 21 CFR Parts 50 and 56. In particular, the informed consent includes a straightforward explanation of the reported increased risks of stroke and vascular complications that have been published for TAVR.
- All aspects of the research study are conducted according to appropriate standards of scientific integrity (see http://www.icmje.org).
- The research study has a written protocol that clearly addresses, or incorporates by reference, the standards listed as Medicare coverage requirements.
- The clinical research study is not designed to exclusively test toxicity or disease pathophysiology in healthy individuals. Trials of all medical technologies measuring therapeutic outcomes as one of the objectives meet this standard only if the disease or condition being studied is life threatening as defined in 21 CFR § 312.81(a) and the patient has no other viable treatment options.
- The clinical research study is registered on the www.ClinicalTrials.gov website by the principal sponsor/investigator prior to the enrollment of the first study subject.
- The research study protocol specifies the method and timing of public release of all prespecified outcomes to be measured including release of outcomes if outcomes are negative or study is terminated early. The results must be made public within 24 months of the end of data collection. If a report is planned to be published in a peer reviewed journal, then that initial release may be an abstract that meets the requirements of the International Committee of Medical Journal Editors (http://www.icmje.org). However a full report of the outcomes must be made public no later than three (3) years after the end of data collection.
- The research study protocol must explicitly discuss subpopulations affected by the treatment under investigation, particularly traditionally underrepresented groups in clinical studies, how the inclusion and exclusion criteria affect enrollment of these populations, and a plan for the retention and reporting of said populations on the trial. If the inclusion and exclusion criteria are expected to have a negative effect on the recruitment or retention of underrepresented populations, the protocol must discuss why these criteria are necessary.
- The research study protocol explicitly discusses how the results are or are not expected to be generalizable to the Medicare population to infer whether Medicare patients may benefit from the intervention. Separate discussions in the protocol may be necessary for populations eligible for Medicare due to age, disability or Medicaid eligibility.
Consistent with section 1142 of the Act, the Agency for Healthcare Research and Quality (AHRQ) supports clinical research studies that CMS determines meet the above-listed standards and address the above-listed research questions.
- We propose national non-coverage of TAVR for all indications other than those noted above, and further specify non-coverage of TAVR in patients with:
- Mixed aortic valve disease (aortic stenosis and aortic regurgitation with predominant aortic regurgitation > 3+);
- Isolated aortic regurgitation;
- Untreated clinically significant coronary artery disease requiring revascularization;
- Hypertrophic cardiomyopathy with or without obstruction;
- Echocardiographic evidence of intracardiac mass, thrombus or vegetation;
- Significant aortic disease, including abdominal aortic or thoracic aneurysm defined as maximal luminal diameter ≥ 5 cm; marked tortuosity (hyperacute bend), aortic arch atheroma (especially if > 5
mm, protruding or ulcerated) or narrowing (especially with calcification and surface irregularities) of the abdominal or thoracic aorta, severe "unfolding" and tortuosity of the thoracic aorta, unless the patient qualifies for a transapical or other aortic or subclavian approaches;
- Iliofemoral vessel characteristics that would preclude safe placement of an introducer sheath such as severe obstructive calcification, severe tortuosity or small vessel size (applicable for transfemoral patients only), unless the patient qualifies for a transapical or other aortic approach.
We are requesting public comments on this proposed determination pursuant to section 1862(l) of the Social Security Act. We are specifically interested in public comments on the use of Coverage with Evidence Development (CED) in this decision. After considering the public comments, we will make a final determination and issue a final decision memorandum.
Appendix A: General Methodological Principles of Study Design
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of an illness or injury or to improve the functioning of a malformed body member. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to help ensure adequate numbers of patients are enrolled to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to which group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is the extent to which differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of which have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
- Randomized controlled trials
- Non-randomized controlled trials
- Prospective cohort studies
- Retrospective case control studies
- Cross-sectional studies
- Surveillance studies (e.g., using registries or surveys)
- Consecutive case series
- Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in which confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to which the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. We are interested in the results of changed patient management not just altered management. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. For most determinations, CMS evaluates whether reported benefits translate into improved health outcomes. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
Appendix B: Tables
From Smith, et al. (NEJM June 9, 2011) Table 2 – Clinical Outcomes at 30 Days and 1 Year in the Intention-to-Treat Population*
| Outcome |
30 Days [Cohort A] |
1 Year [Cohort A] |
| |
Transcatheter Replacement
(N=348) |
Surgical Replacement
(N=351) |
P Value |
Transcatheter Replacement
(N=348) |
Surgical Replacement
(N=351) |
P Value |
| Number of Patients (%) |
Number of Patients (%) |
| Death, from any cause |
12 (3.4) |
22 (6.5) |
0.07 |
84 (24.2) |
89 (26.8) |
0.44 |
| Death, from cardiac causes |
11 (3.2) |
10 (3.0) |
0.90 |
47 (14.3) |
40 (13.0) |
0.63 |
| Repeat hospitalization |
15 (4.4) |
12 (3.7) |
0.64 |
58 (18.2) |
45 (15.5) |
0.38 |
| Death or repeat hospitalization |
25 (7.2) |
33 (9.7) |
0.24 |
120 (34.6) |
119 (35.9) |
0.73 |
| Stroke or TIA |
19 (5.5) |
8 (2.4) |
0.04 |
27 (8.3) |
13 (4.3) |
0.04 |
| TIA |
3 (0.9) |
1 (0.3) |
0.33 |
7 (2.3) |
4 (1.5) |
0.47 |
| Stroke, minor |
3 (0.9) |
1 (0.3) |
0.34 |
3 (0.9) |
2 (0.7) |
0.84 |
| Stroke, major |
13 (3.8) |
7 (2.1) |
0.20 |
17 (5.1) |
8 (2.4) |
0.07 |
| Death, from any cause or major stroke |
24 (6.9) |
28 (8.2) |
0.52 |
92 (26.5) |
93 (28.0) |
0.68 |
| Myocardial infarction |
0 |
2 (0.6) |
0.16 |
1 (0.4) |
2 (0.6) |
0.69 |
| Vascular complication, any |
59 (17.0) |
13 (3.8) |
<0.001 |
62 (18.0) |
16 (4.8) |
<0.001 |
| Vascular complication, major |
38 (11.0) |
11 (3.2) |
<0.001 |
39 (11.3) |
12 (3.5) |
<0.001 |
| Acute kidney injury, Creatinine >3mg/dl (256 umol/liter) |
4 (1.2) |
4 (1.2) |
0.95 |
12 (3.9) |
8 (2.7) |
0.41 |
| Acute kidney injury, renal-replacement therapy
| 10 (2.9) |
10 (3.0) |
0.95 |
18 (5.4) |
20 (6.5) |
0.56 |
| Major bleeding |
32 (9.3) |
67 (19.5) |
<0.001 |
49 (14.7) |
85 (25.7) |
<0.001 |
| Endocarditis |
0 |
1 (0.3) |
0.32 |
2 (0.6) |
3 (1.0) |
0.63 |
| New-onset atrial fibrillation † |
30 (8.6) |
56 (16.0) |
0.006 |
42 (12.1) |
60 (17.1) |
0.07 |
| New pacemaker |
13 (3.8) |
12 (3.6) |
0.89 |
19 (5.7) |
16 (5.0) |
0.68 |
* All percentages are Kaplan-Meier estimates at the specific time point and thus do not equal the number of patients divided by the total number in the study group.
† The presence of new-onset atrial fibrillation was determined in an electrocardiography core laboratory.
From Leon, et al. (NEJM October 21, 2010) Table 2 – Clinical Outcomes at 30 Days and 1 Year*
NA denotes not applicable, TAVI transcatheter aortic-valve implantation, and TIA transient ischemic attack.
| Outcome |
30 Days [Cohort B] |
1 Year [Cohort B] |
|---|
| |
TAVI
(N=179) |
Standard Therapy
(N=179) |
P Value† |
TAVI
(N=179) |
Standard Therapy
(N=179) |
P Value† |
| Number of Patients (%) |
Number of Patients (%) |
| Death, from any cause |
9 (5.0) |
5 (2.8) |
0.41 |
55 (30.7) |
89 (49.7) |
<0.001 |
| Death, from cardiovascular cause‡ |
8 (4.5) |
3 (1.7) |
0.22 |
35 (19.6) |
75 (41.9) |
<0.001 |
| Repeat hospitalization ƒ |
10 (5.6) |
18 (10.1) |
0.17 |
40 (22.3) |
79 (44.1) |
<0.001 |
| Death, from any cause or repeat hospitalization ƒ |
19 (10.6) |
22 (12.3) |
0.74 |
76 (42.5) |
126 (70.4) |
<0.001 |
| Stroke or TIA, all |
12 (6.7) |
3 (1.7) |
0.03 |
19 (10.6) |
8 (4.5) |
0.04 |
| TIA |
0 |
0 |
|
1 (0.6) |
0 |
1.00 |
| Stroke, minor |
3 (1.7) |
1 (0.6) |
0.62 |
4 (2.2) |
1 (0.6) |
0.37 |
| Stroke, major |
9 (5.0) |
2 (1.1) |
0.06 |
14 (7.8) |
7 (3.9) |
0.18 |
| Death, from any cause or major stroke |
15 (8.4) |
7 (3.9) |
0.12 |
59 (33.0) |
90 (50.3) |
0.001 |
| Myocardial infarction, all |
0 |
0 |
|
1 (0.6) |
1 (0.6) |
1.00 |
| Myocardial infarction, periprocedural |
0 |
0 |
|
0 |
0 |
|
| Vascular complications, all |
55 (30.7) |
9 (5.0) |
<0.001 |
58 (32.4) |
13 (7.3) |
<0.001 |
| Vascular complications, major |
29 (16.2) |
2 (1.1) |
<0.001 |
30 (16.8) |
4 (2.2) |
<0.001 |
| Acute kidney injury, Creatinine>3 mg/dl (265 umol/liter)¶ |
0 |
1 (0.6) |
1.00 |
2 (1.1) |
5 (2.8) |
0.45 |
| Acute kidney injury, renal-replacement therapy║ |
2 (1.1) |
3 (1.7) |
1.00 |
3 (1.7) |
6 (3.4) |
0.50 |
| Major bleeding |
30 (16.8) |
7 (3.9) |
<0.001 |
40 (22.3) |
20 (11.2) |
0.007 |
| Balloon aortic valvuloplasty |
1 (0.6)** |
2 (1.1) |
1.00 |
1 (0.6) |
66 (36.9) ††
|
<0.001 |
| Repeat TAVI‡‡ |
3 (1.7) |
NA |
|
3 (1.7) |
NA |
|
| Aortic-valve replacement |
0 |
3 (1.7) |
0.25 |
2 (1.1)** |
17 (9.5) |
<0.001 |
| Endocarditis |
0 |
0 |
|
2 (1.1) |
1 (0.6) |
0.31 |
| New atrial fibrillation |
1 (0.6) |
2 (1.1) |
1.00 |
1 (0.6) |
3 (1.7) |
0.62 |
| New pacemaker |
6 (3.4) |
9 (5.0) |
0.60 |
8 (4.5) |
14 (7.8) |
0.27 |
†P values are for between-group comparisons of the frequency of the event at each time point.
‡ Deaths from unknown causes were assumed to be deaths from cardiovascular causes.
ƒ Repeat hospitalizations were included if they were due to aortic stenosis or complications of the valve procedure (e.g., TAVI).
¶ Patients who received renal-replacement therapy were not included
║Patients who received renal-replacement therapy after randomizations were included.
** One patient in the TAVI group did not receive TAVI (because of failed access) and subsequently underwent balloon aortic valvuloplasty, followed by aortic-valve replacement.
†† A total of 30 patients underwent a repeat balloon aortic valvuloplasty after the index balloon aortic valvuloplasty procedure that had been performed in the first 30 days after randomization, and 36 patients underwent a first balloon aortic valvuloplasty more than 30 days after randomization.
‡‡ Three patients underwent a repeat TAVI within 24 hours after the index TAVI procedure; four patients in the standard-therapy group who underwent TAVI at a nonparticipating site outside the United States are not included here.
Appendix C: January 10, 2012 ACC Letter
January 10, 2012
Louis Jacques, M.D.
Director, Coverage and Analysis Group
Centers for Medicare and Medicaid Services
7500 Security Blvd
Baltimore, Maryland
Dear Louis:
Thanks for the call today and the many helpful interactions with us related to how CMS can develop a coverage policy that allows FDA’s recent approval of TAVR’s to be implemented in the U.S. for Medicare patients. We share mutual interests in having this coverage proceed in a quality, patient-centered, unbiased, safe, and effective fashion.
On behalf of my colleagues at STS, SCAI, AATS, and ACC, I have been asked to forward this information to you. Please understand that the attached should be viewed as preliminary guidance on institutional and individual operator qualifications for performing TAVR, and we want to emphasize that this interim information may change in the official and forthcoming document. Given the urgency for providing information, the attached represents the current best thinking of the four societies’ leaders in these regards, as has been conveyed to the societies from the scientific writing committee as they are finalizing the official document.
Thank you for your leadership in this area, and your agency’s support of both the clinicians and patients who await this new innovation.
Respectfully yours,
Jack Lewin, M.D.
CEO
American College of Cardiology
jlewin@acc.org
202-375-6180
cc: David Holmes MD; Michael Mack MD; Chris White MD; Craig Smith, MD; Rob Wynbrandt JD; Norm Linsky CAE; Elizabeth Dooley Crane
Preliminary Guidance from SCAI, AATS, ACCF and SCAI to CMS – Institutional and Operator Requirements for Transcatheter Aortic Valve Replacement
- For New Centers to be considered for TAVR:
- Institutional Requirements:
-Surgical:
- ≥ 50 total AVR procedures/year, with 10 High Risk (STS Score 6)
- ≥ 2 institutionally-based cardiac surgeons
- Interventional:
- ≥ 400 caths/150 PCIs per year
- ≥ 15 left-sided structural (EVAR,TEVAR, etc.) interventions
- Individual Requirements:
Surgeon:
- Board Certified/Eligible in CV Surgery.
- Professional experience with:
- ≥ 100 AVR/career including 10 high-risk patients OR
- ≥ 25 AVR/Year or 50 AVR in 2 years AND
- ≥ 20 in the last year prior to TAVR
Interventionalist:
- Board Certified/Eligible in Interventional Cardiology
- Professional experience with:
- 50 structural heart disease procedures
- Existing TAVR Centers:
- Participation in ongoing TAVR programs, either PAS or RCTs
- Experience with ≥ 30 TAVR procedures and ≥ to 20 /year
TAVR Program
- ≥ 20 procedures/year OR 40 procedures/2 years
- 30 day all-cause mortality ≤ 15%
- 30 day neurologic events ≤15%
- 30 day major vascular complications ≤15%
- ≥ 90% institutional follow-up
- ≥ 60% one-year survival for non-operable patients
- For all centers either new or established:
- Participation in National TAVR Registry for ongoing enrollment and follow up of all TAVR patients
- Commitment to Heart Team concept
- Adherence to the ACCF/AATS/SCAI/STS expert consensus document on TAVR
[1] 1) Any thoracic aortic dissection; 2) access site or access-related vascular injury leading to either death, need for significant blood transfusions (>3 units), unplanned percutaneous or surgical intervention, or irreversible end-organ damage; 3) distal embolization (non-cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end-organ damage: or 4) left ventricular perforation.


