TO: Administrative File: CAG #00293R2
Microvolt T-wave Alternans
From: Tamara Syrek Jensen, JD
Director, Coverage and Analysis Group
Joseph Chin, MD, MS
Acting Deputy Director, Coverage and Analysis Group
James Rollins, MD, PhD
Division Director
Kim Long, BA
Lead Analyst
Joseph Hutter, MD, MA
Medical Officer
Subject: Proposed Decision Memorandum: Microvolt T-wave Alternans
Date: October 23, 2014
I. Proposed Decision
CMS was asked to reconsider our national coverage determination (NCD) on microvolt T-wave alternans (MTWA) diagnostic testing to extend coverage to the modified moving average (MMA) method. CMS' interest in MTWA testing is in the risk stratification of Medicare beneficiaries who may be at risk for sudden cardiac death (SCD). CMS proposes that there is insufficient evidence to conclude that the MMA method of determining MTWA improves health outcomes for Medicare beneficiaries at risk for SCD and we therefore find that it is not reasonable and necessary under section 1862(a)(1)(A) of the Social Security Act (the Act).
CMS is seeking comments on the proposed decision. We will respond to public comments in a final decision memorandum, as required by §1862(1)(3) of the Act.
II. Background
A. Sudden Cardiac Death
SCD is generally defined as a sudden and unexpected pulseless event due to an underlying primary cardiac event (Chugh 2004, Myerburg 1994). A National Institutes of Health (NIH) work group has provided operational definitions for established and probable SCD (NIH workshop 2009, Fishman 2010). An established case of SCD is an unexpected death without an obvious extra-cardiac cause and which occurred rapidly while being witnessed or within one hour of symptom onset if unwitnessed. Also as mentioned by Fishman, a probable case of SCD is an unexpected death without an obvious extra-cardiac cause and which occurred within the previous 24 hours. SCD can present as ventricular tachycardia (VT), ventricular fibrillation (VF), bradyarrhythmia, asystole, or pulseless electric activity (PEA) (previously called electromechanical dissociation or EMD).
SCD claims 250,000 to 350,000 lives in the United States annually (Lloyd-Jones 2010) with an annual incidence of 50 to 200 per 100,000 in the general population (Albert 2003, Byrne 2008, Cobb 2002, Chugh 2004, de Vreede-Swagemakers 1997, Escobedo 1996, Gillum 1989, Juntilla 2010, Kong 2011, Nichol 2008, Roger 2011, Vaillancourt 2004, Zheng 2001). Although by some estimates, U.S. incidence rates may have declined over time, they remain higher than in Mediterranean countries (Marrugat 1999, Masiá 1998). Ischemic heart disease accounts for approximately 80% of SCD cases in the U.S. (Myerburg 2001) versus about 60% in Mediterranean countries (Subirana 2011). Despite the decline in age-adjusted coronary heart disease mortality, approximately 50% of deaths from coronary heart disease qualify as SCD (Huikuri 2001, Fox 2004). It’s been noted that 40% or more of SCD occurs in those less than 65 years of age and that the majority of SCD events (absolute number) occur in the general population or in the population with no prior cardiac history, but with risk factors for cardiac disease (Chugh 2004, Myersburg 1992). Implantable cardioverter defibrillator (ICD) therapy has been shown to reduce mortality in those who have been resuscitated from near-fatal ventricular arrhythmias and in those who have ischemic or non-ischemic dilated cardiomyopathy, reduced left ventricular ejection fraction and heart failure [see National Coverage Determination (NCD) for Implantable Automatic Defibrillators (20.4)].
B. Electrical Activity of the Heart and T-waves
Electrical activity of the heart can be assessed using the electrocardiogram (ECG). Beat-to-beat changes in the contour, amplitude, or polarity of the T-wave were first described by Hering in 1908 (Hering 1908). The clinical significance of such T-wave alternans remained unknown until Schwartz et al. observed that the T-wave configuration in a patient with syncope and long Q-T syndrome changed with stress and/or physical activity (Schwartz 1975). Changes in T-wave morphology were generally not detectable by visual inspection of the ECG, but these micro T-wave variations could be computed from the power spectrum of the T-wave fluctuations (Adam, 1984).
C. Methods of MTWA Detection
Several methods to detect these T-wave changes have been developed (Martinez 2005). All methods need to (1) locate the T-wave signal by defining a T-wave window, (2) adjust for variation in the baseline of the signal, (3) filter out noise, and (4) align the series of T-waves for superimposition and analysis. Two methods have been approved by the Food and Drug Administration (FDA): the fast-Fourier-transformation spectral analysis (SA) method (Cambridge Heart) and the modified moving average (MMA) method (GE Medical Systems). A technical comparison of these two FDA approved devices appears in our Analysis section below.
III. History of Medicare Coverage
A. Prior Requests
CMS has previously reviewed the scientific literature for MTWA as a diagnostic test for beneficiaries at risk of SCD. On March 21, 2006, CMS established national coverage for MTWA using the SA methodology only. That NCD was based on the SA method's predictive value of who was thought to not likely benefit from ICD therapy (http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=165).
Subsequently, CMS was asked to reconsider the NCD specifically to reverse our current non-coverage of MTWA with the MMA method. On May 12, 2008, CMS determined that there was insufficient evidence to conclude that use of MTWA testing with the MMA method would lead to improved health outcomes for Medicare beneficiaries at risk for SCD (http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=213).
B. Current Request
GE Healthcare has formally requested that CMS reconsider the current MTWA NCD to broaden national coverage to include use of the MMA method as an alternative to the already approved SA method for MTWA testing.
C. Benefit Category
Medicare is a defined benefit program. An item or service must fall within a benefit category as a prerequisite to Medicare coverage §1812 (Scope of Part A); §1832 (Scope of Part B) and §1861(s) (Definition of Medical and Other Health Services) of the Act. MTWA using the MMA method is considered to be within the following benefit category: other diagnostic tests, §1861(s)(3) of the Act. This may not be an exhaustive list of all applicable Medicare benefit categories for this item or service.
IV. Timeline of Recent Activities
| Date |
Action |
| April 23, 2014 |
CMS accepted a formal request for reconsideration of the NCD Manual Section 20.30 to include the MMA method of determining MTWA. A tracking sheet was posted on the web site and the initial 30 day public comment period commenced. |
| May 23, 2014 |
The initial 30 day public comment period ended. Seventeen comments were received. |
| October 23, 2014 |
Proposed decision posted. |
V. FDA Status
The FDA has cleared two methods of MTWA testing through the 501(k) process: Cambridge Heart’s spectral analysis (SA) method and General Electric’s modified moving average (MMA) method. While the focus of this NCD is the MMA method, we discuss both devices for purposes of comparison.
GE’s Modified Moving Average Method
The FDA cleared GE’s initial device for MTWA testing using the MMA method on December 3, 2002 (K023380; www.accessdata.fda.gov/cdrh_docs/pdf2/k023380.pdf ), and cleared the associated algorithm software on October 30, 2003 (K032513; www.accessdata.fda.gov/cdrh_docs/pdf3/k032513.pdf).
The latter clearance document states:
“The T-Wave Alternans (TWA) Algorithm Option is intended for use in a hospital, doctor's office or clinic environment by competent healthcare professionals for recording ST-T wave morphology fluctuations for patients who are undergoing cardiovascular disease testing. T-Wave Alternans (TWA) describes an electrocardiographic (ECG) pattern that exhibits different ST/T-wave morphologies on alternating beats. The algorithm performs the measurement of this variation at an accuracy and resolution of 1-microvolt. The TWA Algorithm Option permits visual confirmation of TWA by displaying the original ECG along with representative complexes made from a moving average of every other beat.
The TWA Algorithm measurements have been found to be predictive of arrhythmic death and can be used for the purposes of risk stratification. The TWA Algorithm Option allows the user to specify the maximal heart rate for valid TWA measurements and the specific heart rate to be attained before TWA is measured. The TWA Algorithm Option is intended to provide only the measurements of the fluctuations of the ST-T-waves. TWA measurements are intended for qualified personnel in evaluating the patient in conjunction with the patient's clinical history, symptoms, other diagnostic tests, as well as the professional's clinical judgment. No interpretation is generated.”
The FDA cleared an ancillary digital ambulatory ECG recorder on September 2, 2005 (K042782; www.accessdata.fda.gov/cdrh_docs/pdf4/k042782.pdf).
Cambridge Heart’s Spectral Analysis Method
The FDA cleared high resolution, noise reducing ECG electrodes (K962115, 8/29/1996, www.accessdata.fda.gov/cdrh_docs/pdf/K962115.pdf and K002230, 8/18/2000, www.accessdata.fda.gov/cdrh_docs/pdf/K002230.pdf) and the initial versions of the CH 2000 Cardiac Diagnostic System to collect and record electrocardiographic data (CH 2000 Stress Test System, K950018, 2/29/1996 and CH 2000 Cardiac Diagnostic System [modified], K981697, 10/14/1998, www.accessdata.fda.gov/cdrh_docs/pdf/K981697.pdf.)
The FDA cleared the first computer processing system for T-wave alternans as a system that measured T-waves without other ECG components (K001034, 6/9/2000, www.accessdata.fda.gov/cdrh_docs/pdf/K001034.pdf) and then as HearTwave (K003492, 1/18/2001 www.accessdata.fda.gov/cdrh_docs/pdf/K003492.pdf). The latter clearance document states:
“The Alternans Processing System used with the Analytic Spectral Method of Alternans Processing is intended for the measurement of Microvolt T-wave Alternans at rest and during ECG stress testing.
The presence of Microvolt T-wave Alternans as measured by Analytic Spectral Method of Alternans Processing in patients with known, suspected or at risk of ventricular tachyarrhythmia predicts increased risk of a cardiac event (ventricular tachyarrhythmia or sudden death).
The Analytic Spectral Method of Alternans Processing should only be used as an adjunct to clinical history and the results of other non-invasive and/or invasive tests.
The predictive value of T-wave Alternans for cardiac events has not been established in patients with active untreated ischemia.
Microvolt T-wave Alternans is defined by T-wave Alternans, which is
a) measured by high resolution sensors,
b) is present in leads X,Y, Z, VM or two adjacent precordial leads,
c) is at the level of 1.9 microvolts after signal optimization and subtraction of the background noise level,
d) is at least three standard deviations greater than the background noise level,
e) is present at rest or has an onset heart rate of below 110 beats per minute, and
f) is sustained at heart rates above the onset heart rates.”
“The Alternans Processing System works in conjunction with a host standard-stress ECG controller. In the Cambridge Heart Model CH 2000 Cardiac Diagnostic System, the host controller is integral to the device (K983102)... The Alternans test using the Alternans Processing System is performed with seven standard stress test electrodes and seven proprietary multi-segment Micro-V Alternans Sensors.”
In a later clearance document (K012206, 10/12/2001, www.accessdata.fda.gov/cdrh_docs/pdf/K012206.pdf), the computer processing system was reported to have utility “at rest and during treadmill, ergometer, electrophysiological, and pharmacologic testing” and that the system uses interpretive Alternans Report Classifier software that provides an assessment of the alternans report data to assist the physician in diagnosis. The computerized assessment is printed at the bottom of the alternans report and indicates that the result is consistent with Positive, Negative, or Indeterminate finding.”
This processing system was more fully integrated into the Cambridge devices for collecting and recording electrocardiographic data in subsequent clearances.
VI. General Methodological Principles
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member consistent with §1862(a)(1)(A). Critical appraisal of the evidence enables CMS to determine to what degree CMS is confident that: (1) the specific assessment questions can be answered conclusively; and (2) the intervention will improve health outcomes for beneficiaries. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
A detailed account of the methodological principles of study design that the Agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A.
Public commenters sometimes cite the published clinical evidence and provide CMS with useful information. Public comments that provide information based on unpublished evidence, such as the results of individual practitioners or patients, are less rigorous and, therefore, less useful for making a coverage determination. CMS uses the initial comment period to inform the public of its proposed decision. CMS responds in detail to the public comments that were received in response to the proposed decision when it issues the final decision memorandum.
VII. Evidence
A. Introduction
The evidence reviewed includes the published medical literature on pertinent clinical studies using the MMA method for MTWA testing.
B. Discussion of Evidence Reviewed
1. Key Question
Is the evidence sufficient to conclude that MTWA testing using the MMA method improves health outcomes for Medicare beneficiaries who are candidates for ICD placement?
2. External Technology Assessments
CMS did not request an external technology assessment (TA) on this issue.
3. Internal Technology Assessment
The evidence review included: (1) articles and reports submitted by the requestor as well as those obtained by searching literature and technology review databases from PubMed, EMBASE, the Agency for Healthcare Research and Quality (AHRQ), the Blue Cross/Blue Shield Technology Evaluation Center, Cochrane, and the National Institute for Health and Care Excellence (NICE); (2) FDA assessments and surveillance reports; (3) NIH workshop proceedings; and (4) published professional society commentary (which ranged from informed opinion to formal evidence-based analysis). We excluded oral presentations, unpublished white papers, abstracts, case reports, non-English publications and studies with fewer than 100 patients to ensure that studies were peer-reviewed and sufficiently powered. CMS also searched Clinicaltrials.gov to help identify relevant clinical trials.
i. Requestor Submission
To support its request for coverage, the requestor initially submitted 32 papers as PDFs or bibliographic citations.
ii. CMS Literature Search Terms
The search terms used by CMS were:
- Sudden cardiac death, cardiac arrest, resuscitation, and syncope.
- T-wave alternans alone or combined with arrhythmia, anti-arrhythmic drugs, ß-blockers, cardiac defibrillator, cardiomyopathy/cardiac myopathy, dysrhythmia, ejection fraction, fibrillation, heart failure, implantable cardioverter defibrillator (ICD), infarction, primary prevention, risk stratification, sudden (cardiac) death, ventricular tachycardia, Alternans Before Cardioverter Defibrillator (ABCD), Multicenter Automatic Defibrillator Implantation Trial II (MADIT II), Microvolt T Wave Alternans Testing for Risk Stratification of Post MI Patients (MASTER), Risk Estimation Following Infarction, Noninvasive Evaluation (REFINE), and Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT).
iii. CMS Search Results
Our goal was to find clinical trials that linked diagnostic results of the MTWA test using the MMA method with the selection of a therapeutic intervention that improved health outcomes - especially for those at risk of SCD. Based on the search methods and inclusion and exclusion criteria stated above, we found 21 publications constituting 15 unique clinical studies (see Table 1). The investigators of The Finnish Cardiovascular Study (FINCAVAS) presented serial data on an ever-larger patient cohort accrued over time (Leino 2009, Leino 2011, Minnikinen 2009, Nieminen 2007, Slawnych 2009). Surgit evaluated patient populations in two studies that appeared to overlap (Surgit 2014a, Surgit 2014b). One publication is an ancillary analysis of combinations of previously published studies (Slawnych 2009).
Patient Populations
Investigators evaluated the utility of MTWA testing in a variety of patient populations, including those status post myocardial infarction, those with left ventricular dysfunction including heart failure, those with congenital heart disease, those with hypertension, and a community dwelling geriatric population. However, no investigators studied populations that have established indications for implantation of a cardiac defibrillator.
Study Design
There were no randomized controlled trials. Study designs were prospective, retrospective or cross-sectional analyses. Blinding, where present, was limited to those interpreting test results or adjudicating causes of death. Six studies used controls. Of these, four used matched controls (Kenttä 2014, Nieminen 2014, Stein 2008, 2010), one used a cohort control (Li-na 2012), and one study used normal subjects as controls (Trojinarska 2014).
The remaining studies conducted intra-group comparisons. Six studies compared differences in clinical outcomes on the basis of T-wave alternans status (Exner 2007, FINCAVAS series, Hoshida 2013, Yu 2012, Kim 2014, Sakaki 2009). Six studies compared the differences in the likelihood of aberrant T-wave alternans on the basis of the presence or absence of dysrhythmic outcome or death (Arisha 2013, FINCAVAS series, Kim 2014, Sulimov 2012, Sakaki 2009, Yu 2012).
Three of the studies with matched controls divided patients on the basis of clinical outcomes (Nieminen 2014, Stein 2008, 2010). Four publications (with three unique study populations) described differences in MTWA by patient cohort (Li-na 2011, Surgit 2014a, Surgit 2014b, Trojinarksi 2014) but not hard outcomes.
No studies were designed to control for the confounding introduced by the selective use of ICD either at study entry or subsequent implantation (see Table 1B).
Study Endpoints
Investigators utilized a variety of primary endpoints:
- SCD alone or in combination with any of the following: cardiac arrest/resuscitation, cardiac death, all-cause mortality, PVCs, sustained ventricular tachycardia, or ventricular fibrillation (Arisha 2013, Yu 2012);
- cardiac death alone or in some combination with cardiac arrest or resuscitation, or all-cause mortality (Exner 2007, Hoshida 2013, Li-na 2012, Sakaki 2009, Slawnych 2009);
- death alone or in combination with transplantation (Kim 2014, Stein 2010);
- change in MTWA status (Kenttä 2014);
- MTWA status in those with SCD, cardiac death, or all-cause mortality (Nieminen 2014, Stein 2008);
- differences in MTWA status (Li-na 2011, Surgit 2014a, Surgit 2014b, Trojinarksa 2014).
For some studies, the primary endpoint was not clearly stated:
- in their review of post exercise T-wave alternans test results from the REFINE and FINCAVAS studies, Slawnych et al. delineated both all-cause mortality and cardiovascular mortality as the primary endpoint (Slawnych 2009);
- in the FINCAVAS study series, the publications Leino 2011, Nieminen 2007, Minkkinen 2009 used SCD, cardiovascular death and all-cause as primary endpoints, and Leino 2009 publication used cardiovascular death and all-cause mortality as primary endpoints;
- in the Nieminen 2014 publication, endpoints included SCD, cardiovascular death, all-cause death, and four or eight beat series of ventricular tachycardia on day one of hospitalization.
Study Results
Eight investigator groups using a variety of endpoints reported positive results supportive of MTWA use in their longitudinal clinical studies:
- Hoshida 2013, post myocardial infarction, n=313, mean age=70 years, LVEF=47%;
- Yu 2012, post myocardial infarction 1-15 days, n=227, mean age=56 years, LVEF≥ 35% in 199 patients;
- Leino 2011, referred for exercise study, n=3598, mean age=55.6 years, LVEF not reported;
- Li-na 2012, myocardial infarction, n=96 cases, 75 controls, mean age=65 years in cases, LVEF=45% or higher;
- Sakaki 2009, mixed ventricular dysfunction, n=295, mean age=66 years, LVEF=34%;
- Stein 2008, post myocardial infarction, n=92 cases, 46 controls, mean age=66 years, LVEF=34% in cases;
- Stein 2010, healthy community dwelling, n=, 49 cases, 98 controls, mean age=73 years, LVEF normal in 108 participants;
- Sulimov 2012, post myocardial infarction, n=111, mean age=64.1 years, mean LVEF=46.6%.
Two investigator groups reported mixed results regarding the utility of MTWA:
- Exner et al. reported positive results when MTWA testing was conducted 10 to 14 weeks after myocardial infarction, but negative results when testing was conducted 2 to 4 weeks post myocardial infarction; n=322 (Exner 2007). Both MMA and SA data were apparently collected but a comparative analysis was never reported.
- Nieminen et al. reported equivocal results in patients with non-ST segment elevation acute coronary syndrome. Elevated MTWA values were linked with total mortality, but not with SCD or total cardiac mortality in a sub-analysis of a drug therapy trial potentially confounding the findings (Nieminen 2014).
Four investigator groups reported negative results for their clinical trials:
- Arisha et al. reported that different MTWA power results depending on the channel and that result predicted outcomes (Arisha 2013). The investigators also found that the separate method of heart rate turbulence predicted outcomes and that prediction could be improved when combined with measures of left ventricular function and MTWA status.
- Kim et al. reported that MTWA results did not predict all-cause mortality or cardiac transplantation (Kim 2014).
- Li-na et al. reported higher MTWA power levels in patients with diabetes and a history of myocardial infarction than in patients without diabetes, but with a history of myocardial infarction. The combination of heart rate turbulence and MTWA status predicted future cardiac mortality, but MTWA status alone did not (Li-na 2012).
- Kenttä et al. reported small reductions in MTWA levels, but no reductions in cardiac mortality, after a two-year exercise program for patients with angiographically-confirmed coronary artery disease (Kenttä 2014).
Review Summary
Of the 21 publications found, 15 were unique clinical studies. Of these, 10 studies reported positive results. There were no randomized controlled trials. Only the FINCAVAS series studied 500 or more patients. Only two studies (one positive, one negative) used SCD or resuscitated cardiac arrest as a primary endpoint. No studies assessed MTWA testing in patient populations limited to the type in which ICD therapy has been demonstrated to reduce mortality. No studies used complete blinding. Although the focus of our evidence review was on the MMA method, we did search for head-to-head comparative studies between SA and MMA, and found none.
4. MEDCAC
A Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) meeting was not convened on this issue.
5. Evidence-based Guidelines
No guideline definitively recommends use of MTWA test results to determine subsequent therapeutic intervention, including that of ICD placement, nor were there any guidelines that specifically focused on MMA or compared MMA to SA.
a. (2010) Sudden Cardiac Death Prediction and Prevention Report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3016224/pdf/nihms258535.pdf
Fishman et al. 2010
“In contrast to invasive electrophysiological testing, noninvasive tests for predicting SCD are clearly more attractive in a clinical strategy for widespread screening. Numerous markers derived mainly from surface ECG have been correlated with SCD, cardiac, and total mortality over the past 3 decades. These can be classified as (1) indices of abnormal autonomic modulation of cardiovascular function such as heart rate variability, heart rate turbulence, heart rate recovery from exercise, and baroreflex sensitivity; (2) indices of abnormal impulse conduction such as signal averaged ECG and QRS fractionation; and (3) indices of abnormal repolarization such as microvolt T wave alternans, QT interval dynamicity, and various measures of T wave morphology and dispersion.
Most of the autonomic markers have been correlated with total rather than arrhythmic mortality. Although extensive comparative data are not available, when examined in the same population with other risk markers T wave alternans appear to predict SCD-related events with greatest negative predictive value, suggesting that a patient with systolic dysfunction and a negative T wave alternans test may be at comparatively low risk for events. However, other recently published data from 2 large clinical trials of the prophylactic ICD indicate that the use of T wave alternans is likely to be limited by low predictive ability, higher number of indeterminate tests, and concern about incremental value over known risk factors. Taken together, the available experience suggests that multiple risk markers used in combination may provide a more robust prediction of events, which is not surprising when one considers the complexity and diversity of electro-anatomic substrates that underlie SCD.” To date, no randomized clinical trials have been conducted that demonstrate benefit of non-invasive risk stratification in reducing SCD events.
However, the overwhelming majority of SCDs occurs in the general population, and approximately 55% of men and at least 68% of women have no clinically recognized heart disease prior to SCD.
Current methods to differentiate patients at highest risk for arrhythmic death from all-cause death are insufficient and lack robustness in guiding the use of ICD therapy. Data on SCD risk are best developed in patients with moderate or severe LV dysfunction either after MI or with chronic ischemic or nonischemic cardiomyopathies. Although patients without severe systolic dysfunction are at lower individual risk, many sudden deaths occur in such patients. Strategies for effective risk stratification in these moderate risk populations should be investigated. The optimal approaches for combining potential risk factors to identify individuals at risk and to target risk factors for treatment have not been determined.”
b. (2011) Microvolt T-Wave Alternans: Physiological Basis, Methods of Measurement, and Clinical Utility—Consensus Guideline by International Society for Holter and Noninvasive Electrocardiology (Cosponsored by the Japanese Circulation Society, the Computers in Cardiology Working Group on e-Cardiology of the European Society of Cardiology, and the European Cardiac Arrhythmia Society) Verrier et al. 2011
http://dx.doi.org/10.1016/j.jacc.2011.06.029
"In their meta-analysis, Hohnloser et al. proposed a clinical algorithm to identify patients who would not benefit from ICD implantation for primary prevention (see their Fig. 2.) Their analysis revealed that the mortality rate of TWA negative patients with LVEF <=35% but no history of ventricular arrhythmias and no prior ICD implantation was 4-fold lower than that of MADIT II or SCD-HeFT trial patients randomized to ICD therapy. Accordingly, the negative predictive value derived for this group is >99%. This algorithm will require testing in a prospective trial."
“Interventional trials, which have been performed to date only with the Spectral Method, have not demonstrated that a negative TWA test result can sufficiently guide decision-making with regard to ICD implantation.”
“...Frontiers of TWA research include use in arrhythmia risk stratification of individuals with preserved ejection fraction, improvements in predictivity with quantitative analysis, and utility in guiding medical as well as device-based therapy. Overall, although TWA appears to be a useful marker of risk for arrhythmic and cardiovascular death, there is as yet no definitive evidence that it can guide therapy.”
6. Professional Society Position Statements
No professional societies submitted letters during the initial comment period.
7. Expert Opinion
We did not solicit expert opinions on the use of MTWA using the MMA method.
8. Public Comments
Initial Comment Period: April 23, 2014 – May 23, 2014
CMS received 17 timely public comments during the first public comment period. Thirteen of these comments supported expanding CMS coverage to include the MMA method of MTWA testing. They stated that it is advantageous to cover both options since they provide complimentary results. One comment supported CMS considering alternatives to the SA method but did not directly comment on the MMA method. Three comments did not support coverage of the MMA method. They stated that there is a lack of prospective data and that no new clinical data has become available since the last reconsideration.
Of the 17 comments received, four were from cardiologists, four were from professors of medicine, two were from professors of cardiology, one was from a professor of biomedical engineering, two were from medical directors (one for clinical research and the other for a lab that tests heart rate variability (HRV)), one was from www. alternans.org (a European news website), one was from the requestor, one was from the manufacturer of the SA method, and one was an individual who did not identify with an associated organization or profession. Several of the comments cited references, many of which we had already reviewed. The additional references have been considered in our evidence review for this proposed decision.
Full text public comments without personal health information can be viewed at http://www.cms.gov/medicare-coverage-database/details/nca-view-public-comments.aspx?NCAId=275
VIII. CMS Analysis
A. General
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1869(f)(1)(B) of the Act). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, items or services must be "reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member." (See §1862(a)(1)(A) of the Act. This section presents the Agency’s evaluation of the evidence considered and conclusions reached for the assessment).
A diagnostic test is not expected to directly change health outcomes. Rather, a diagnostic test affects health outcomes through changes in disease management brought about by physician actions taken in response to test results. Such actions may include decisions to treat or withhold treatment, to choose one treatment modality over another, or to choose a different dose or duration of the same treatment. Thus, in the case of MTWA we expect that the evidence base would demonstrate changes in patient outcomes by informing physician decisions about which beneficiaries may need an ICD and which do not. The Medicare regulations at 42 CFR 410.32(a) state in part, “…diagnostic tests must be ordered by the physician who is treating the beneficiary, that is, the physician who furnishes a consultation or treats a beneficiary for a specific medical problem and who uses the results in the management of the beneficiary’s specific medical problem.” In this instance we focus on evidence to determine if MTWA testing by the MMA method correctly stratifies Medicare beneficiaries’ risk for SCD and thus informs the treating physicians’ decision to recommend or not recommend ICD placement.
B. Answer to Key Question:
Is the evidence sufficient to conclude that MTWA testing using the MMA method improves health outcomes for Medicare beneficiaries who are candidates for ICD placement?
The answer to this question is "No." Our evidence review found 15 unique clinical studies out of 21 relevant publications. There was no prospective randomized controlled trial (RCT) examining the MMA method, nor any prospective head-to-head comparative study of the MMA and SA methods. There were no high quality studies that addressed the analytical question of whether MTWA testing using the MMA method improves health outcomes for Medicare beneficiaries who are candidates for ICD placement (see NCD 20.4 at: http://cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=110&ncdver=3&NCAId=148&bc=AiAAAAAAAgAAAA%3d%3d&). Of the 15 studies, ten reported positive results; however, seven of these were conducted outside the U.S. in populations that were generally younger and healthier in terms of number and severity of comorbidities than the Medicare population. One sub-analysis (Nieminen 2014) was limited by confounding and two other studies (Stein 2008, Stein 2010) were small, exploratory case-control studies with no subsequent confirmatory studies.
There is insufficient evidence to conclude that the two methods of MTWA testing, SA and MMA, are substantially comparable. As we discuss extensively below, these are different technologies that operate with different technical parameters based on fundamentally different approaches (and this is consistent with FDA’s treatment of them as separate devices). There appears to be considerably less standardization in test parameters (microvolt thresholds, which leads to interpret, etc.) among studies using the MMA method compared to those using the SA method. Most importantly, as stated above, there are no prospective head-to-head comparative studies of the two methods. One study (Exner 2007) included both methods but comparative results were not reported. Thus we cannot confidentially impute results of studies using the SA method to the MMA method.
Other concerns raised in our 2008 NCD still remain. The previous issues related to simulations and non-clinical outcome measures has been removed, since both simulation models as well as animal studies do not provide a sufficient degree of evidence. But important issues persist. First, study samples generally remain small and/or do not adequately represent the U.S. Medicare population. Several of the publications (Exner, 2007; Leino, 2009; Leino, 2011; Minnikinen, 2009; Nieminen, 2007; Slawnych, 2009) on two of the larger studies (REFINE, FINCAVAS) were conducted outside the U.S. The population in the largest study (FINCAVAS; n=500) was highly selective. For example, all patients not meeting the exercise criteria (heart rate over 125 during exercise) were excluded. The FINCAVAS patients were younger and healthier (and likely had fewer co-morbidities) than Medicare beneficiaries. The study exclusions combined with sociodemographic differences among countries makes it difficult to generalize these studies to the Medicare population. Generalizability goes to the external validity of clinical trial evidence and is an established criteria for NCDs. And unfortunately there were no subsequent confirmatory studies conducted in the U.S. The two published studies performed at U.S. centers (Stein 2008; Stein 2010) were small case-control studies. Case-control studies, in general, may help generate hypotheses for further research, but are not conclusive in themselves.
This touches on our second concern: there continues to be a lack of adequate control groups generally. Of the eight positive studies, three used matched controls (Nieminen 2014, Stein 2008, 2010). No studies were designed to control for the confounding introduced by the selective ICD use or drug therapy (Nieminen 2014).
Finally, because of the small sample sizes and low event rates, the positive and negative predictive values of the test are not well- established. We found only three studies that reported predictive values; the results were variable and hence inconclusive).
In sum, based on a thorough review of the studies published since 2008 on MTWA, we propose that the evidence still does not satisfactorily address our study concerns regarding small sample size, generalizability to the Medicare population, and lack of control groups. Most importantly, there is insufficient evidence to conclude that MTWA testing using the MMA method improves health outcomes for Medicare beneficiaries who are candidates for ICD placement. We find MTWA testing using the MMA method is not reasonable and necessary and propose to continue non-coverage.
C. Technical Comparison of MMA and SA Methods
As discussed in the Background section of this PDM, several methods to detect MTWA have been developed (Martinez 2005). All methods need to (1) locate the T-wave signal by defining a T-wave window, (2) adjust for variation in the baseline of the signal, (3) filter out noise, and (4) align the series of T-waves for superimposition and analysis. Our focus is on the SA and MMA methods as these are the only two that are FDA approved. CMS covers the SA method of MTWA analysis, and the request to re-open our NCD explicitly invited comparison of the two methods.
We discussed above (part B of this CMS Analysis section) that there is insufficient evidence to conclude that the SA and MMA methods are substantially comparable technologies. This section provides further detail on the technical comparison.
1. The Spectral Analysis (SA) Method
The SA method for MTWA testing first aligns the ECG beats. Then the differences in the amplitudes of corresponding points in consecutive ST-T segments are measured and used to generate beat-to-beat series of amplitude fluctuations. This information is then subjected to spectral analysis using fast Fourier transformation which averages the differences across the entire ST-T segment (or JT interval). The alternating beat oscillation is 0.5 units of cycles per beat (half the heart rate) and is referred to as the alternans frequency. Test results are reported as the alternans power level and as the T-wave alternans ratio. The alternans power, measured in microvolts (μV) is the difference between the power at the alternans frequency and the power at an adjacent frequency band which serves as a reference point to measure noise. A power level of 1.9 μV or greater is consistent with the presence of T-waves. The T-wave alternans ratio is the number of standard deviations by which the peak signal of the T-wave exceeds the background noise, or the ratio of alternans power divided by the standard deviation of the noise in the reference frequency band. Ratios of three or greater are considered to be consistent with the presence of T-waves whereas an absent alternans ratio is considered to be a negative result. Values in between are considered indeterminate. The SA method requires specialized software and equipment, including proprietary cardiac electrodes (“leads”).
As noted in the FDA-approved label for the SA method (see FDA Status, section V of this PDM), both the protocols for performing the MTWA test with the SA method, and interpretation of test results, are standardized.
2. The Modified Moving Average (MMA) Method
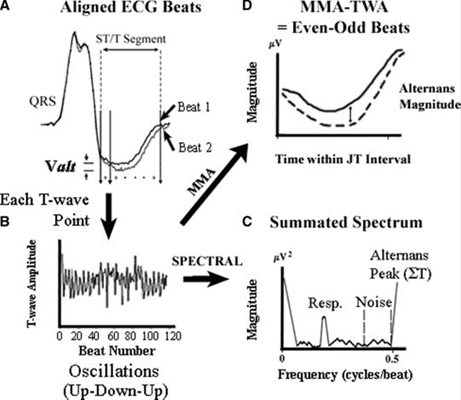
The MMA method for MTWA testing uses a “temporal domain” in which T-waves alternans are assessed as a continuous variable along the complete ECG (see Figure 1). The method divides a series of beats (often 8 or 32) into even and odd compartments. The morphology of the beats in each compartment is processed as a running average. This updating limits the contribution size of any given incoming beat, including aberrant beats. Furthermore, a non-linear filter is applied to every new beat in the running average to reduce sporadic artifacts. The resulting T-wave alternans magnitude (power level) is calculated as the maximum difference in amplitude between the even-beat and odd-beat average in ST-T segments (or JT intervals). The T-wave alternans magnitude as measured by the MMA method appears to be larger (20–70 μV) than that measured by the SA method, which averages the difference across the entire JT interval. Unlike the SA method, the MMA method of MTWA testing does not use specialized electrodes.
Compared to the SA method, the MMA method is relatively non-standardized in terms of how data is collected (investigators have used various combinations of ECG leads) and how results are interpreted (there is no established voltage level indicative of a pathologic test result, and voltage cut-points appear to vary depending on the population under study).
C. Health Disparities
The subjects in key clinical trials evaluating the utility of MTWA testing in patients with left ventricular dysfunction were predominantly male (~75-85%). The proportion of women was higher in studies of advanced heart failure whether ischemic and non-ischemic in origin (~30%) and in studies for other clinical indications (~40%) (FINCAVAS series). This finding, in part, reflects the prevalence and earlier onset of coronary heart disease in men.
Racial and ethnic information was not included in the majority of studies. This lack of evidence about racial and ethnic factors is an evidence gap and may be clinically important given the potential for more T-wave alternans aberrancy in non-Caucasians.
None of the clinical trials included demographic information on other population classifiers historically associated with healthcare access or outcome disparities, such as religion, ethnicity, and sexual orientation.
D. Analysis Summary
There are no published high quality studies that directly address the research question posed in this National Coverage Analysis (NCA). There was no prospective randomized controlled trial examining the MMA method, nor any prospective head-to-head comparative studies of the MMA and SA methods. There is insufficient evidence to conclude that the two methods of MTWA testing, SA and MMA, are substantially comparable. These are different technologies that operate with different technical parameters based on fundamentally different approaches. Moreover, there is considerably less standardization in test parameters (microvolt thresholds, which leads to interpret, etc.) among studies using the MMA method compared to those using the SA method. Finally, the published evidence is not readily generalizable to the Medicare population.
IX. Conclusion
CMS was asked to reconsider our national coverage determination (NCD) on microvolt T-wave alternans (MTWA) diagnostic testing to extend coverage to the modified moving average (MMA) method. CMS' interest in MTWA testing is in the risk stratification of Medicare beneficiaries who may be at risk for sudden cardiac death (SCD). CMS proposes that there is insufficient evidence to conclude that the MMA method of determining MTWA improves health outcomes for Medicare beneficiaries at risk for SCD and we therefore find that it is not reasonable and necessary under section 1862(a)(1)(A) of the Social Security Act (the Act).
CMS is seeking comments on the proposed decision. We will respond to public comments in a final decision memorandum, as required by §1862(1)(3) of the Act.
Appendices
APPENDIX A
General Methodological Principles of Study Design (Section VI of the Decision Memorandum)
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that:
1) the specific assessment questions can be answered conclusively; and
2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages:
1) the quality of the individual studies;
2) the generalizability of findings from individual studies to the Medicare population; and
3) over-arching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes, to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to that group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is to the extent that differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well-designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of that have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
- Randomized controlled trials
- Non-randomized controlled trials
- Prospective cohort studies
- Retrospective case control studies
- Cross-sectional studies
- Surveillance studies (e. g., using registries or surveys)
- Consecutive case series
- Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors.
For observational, and in some cases randomized controlled trials, the method in that confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered, but would suffer from limited generalizability.
The extent to that the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.
Appendix: Table 1 and Figure 1
Table 1A Studies Using Modified Moving Average Method
| Post Myocardial Infarction (MI) |
Prospective |
Controlled |
Randomized |
Blinded |
Study Objective |
Endpoint |
| Arisha 2013 |
Yes |
W/in group comparison by outcome ± LVEF |
No |
No |
Utility of TWA collected by channel V1 vs V2, turbulence onset, and turbulence slope |
SCD, sustained VT/VF requiring cardioversion, ∑mortality, |
Exner 2007
REFINE
Multi-center Canada
See Spectral
|
Yes |
W/in group comparison by TWA status ± other tests |
No |
Yes for TWA & other test readers & death ad-judication |
Utility of TWA, SAECG for QRS duration), Holter for PVCs, heart rate variability & turbu-lence, baroreflex sensitivity using phenylephrine, LVfxn.
Comparison of spectral & MMA.
Comparison of testing at ~3 wks to ~12 wks p MI.
|
Resusc arrest, CD |
Slawnych 2009*
REFINE
Only p exercise data for MMA
|
This was a post hoc retrospective analysis compared/ combined w results from another study |
W/in group comparison by TWA status |
No |
Yes for TWA readers & death ad-judication in the initial trial |
Utility of TWA |
SCD ∑mortality, cardiac mortality
See above. |
Hoshida 2013 |
Yes |
W/in group comparison by mortality status |
No |
No |
Utility of turbulence, TWA |
SCD, cardiac mortality, VF, (non-)sustained VT
Non-cardiac death excluded |
Yu 2012 |
Yes |
W/in group comparison by mortality status |
No |
Yes |
Utility of TWA (+ & frequency), NSVT, PVCs |
SCD or resuscitation |
Nieminen 2013**
MERLIN-TIMI subset
Multi-center International
|
No |
Compared pts w ≥4 beat VT or SCD to match-ed pts w/o. Also compared by TWA level. |
No (MERLIN randomized to ranolazine or placebo) |
Yes for
TWA readers & death ad-judication in the initial trial |
Utility of TWA (done ~d 2 & d6) & parts of TIMI score |
Stated as SCD, CD, ∑mortality, but is traits included TWA scores of those ±those conditions |
| Li-na 2012 |
Yes |
MI+DM
MI w/o DM
W/o CVD
|
No |
No |
Utility of turbulence, TWA (+ & peak) |
Cardiac death
(Pts w other death excluded from analysis)
|
Stein 2008***
EPHESUS subset
Multi-center International
|
No |
Nested case control. Match-ed to SCD/CVD cases 2:1. |
No (EPHESUS randomized to eplerenone or placebo) |
No |
Utility of TWA-including by time of day, heart rate at time of maximal TWA
MMA vs Spectral in n=41
(Limited information) |
Characteristics of those who died of SCD, OCD, or who survived. |
| Sulimov 2012 |
Yes |
Yes no CAD
W/in group comparison by mortality status |
No |
No |
C LVF
TWA in 24 hrs, 100 bpm, at 5AM |
SCD
∑mortality, cardiac mortality
PVCs, VT |
| ↓ Left Ventricular Function |
| Sakaki 2009
(mixed ischemic & non-ischemic) |
Yes |
W/in group comparison by TWA status & mortality status |
No |
No |
Utility of TWA |
Cardiac mortality and SCD |
| Heart Failure |
| Kim 2014
1 center US |
Yes |
W/in group comparison by MA & TWA status |
No |
No |
Utility of MA and TWA |
∑mortality, heart transplant
|
| Exercise Test Referral |
Leino 2011****
FINCAVAS |
Yes |
W/in group comparison by TWA status & mortality status |
No |
No |
Utility of TWA using different lead combinations |
SCD, ∑mortality, cardiac mortality |
Minnikinen 2009
Subset of Leino 2011
1 site Finland |
Yes |
W/in group comparison by TWA status & mortality status |
No |
No |
Utility of TWA at various voltage cut-points |
SCD, ∑mortality, cardiac mortality
|
Leino 2009
Subset of Leino 2011 |
Yes |
W/in group comparison by mortality status |
No |
No |
Utility of TWA at 2 voltage cut-points, heart rate recovery during the minute post exercise |
∑mortality, cardiac mortality |
Nieminen 2007
Subset of Leino 2011
1 site Finland |
Yes |
W/in group comparison by TWA status & mortality status |
No |
No |
Utility of TWA at various voltage cut-points |
SCD, ∑mortality, cardiac mortality |
Slawnych 2009
Subset of Nieminen 2007
Part of a paper combining REFINE & FINCAVAS |
No
Post hoc subset |
W/in group comparison by TWA status & mortality status |
No |
No |
Utility of TWA at 2 voltage cut-points in the post exercise recovery period |
∑mortality, cardiac mortality |
| Angiography Referral |
Kenttä 2014
ARTEMIS substudy
1o study publication cannot be found |
No |
Matched controls |
No
Reportedly 1o study randomized, but no publication can be found |
Yes for
TWA readers |
Utility of TWA |
Change in TWA w exercise |
| Hypertension |
| Surgit 2014a
1 center Turkey |
Yes |
Yes, by left ventricular geometry |
No |
No |
Utility of TWA |
TWA differences in normal geometry, concentric remodeling, concentric hypertrophy, and eccentric hypertrophy |
| Surgit 2014b
1 center Turkey |
Yes |
Yes, by blood pressure status |
No |
No |
Utility of TWA |
TWA differences w normal blood pressure and w hypertension ± circadian rhythm |
| Ambulatory Elderly |
| Stein 2010
Cardiovascular Health Study subset |
No |
Nested case control. Matched to SCD cases. |
No |
No |
Utility of HR variability, turbulence, peak TWA |
Differences in HR variability, turbulence, TWA, PVCs alone or in combination.
Time to death if any |
* The REFINE study employed both Spectral and MMA methods for analysis of TWA. Slawnych et al. presented data from the REFINE and FINCAVAS studies. Only the post exercise data using the MMA method were compared. (Slawnych 2009)
**MERLIN-TIMI=Metabolic Efficiency With Ranolazine for Less Ischemia in Non−ST-Elevation Acute Coronary Syndromes-Thrombolysis In Myocardial Infarction (Ranolazine is an inhibitor of the slowly inactivating component of the cardiac sodium current that did not reduce cardiovascular events in the study.)
***EPHESUS=Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (Eplerenone is a selective aldosterone antagonist that reduced mortality in the study)
ARTEMIS=Innovation to Reduce Cardiovascular Complications of Diabetes at the Intersection Study
****FINCAVAS=Finnish Cardiac Study
Table 1B Studies Using Modified Moving Average Method
| Post Myocardial Infarction (MI) |
Inclusion Criteria |
Exclusion Criteria |
ß-Blocker |
Stratified by |
| NYHA or EF |
DM |
|---|
| Arisha 2013 |
MI, ST segment elevation in 2 leads & ↑ troponin
(Could have PCI w this MI; 92%)
ICD implantation permitted |
LBBB
Non-sinus rhythm
Prior MI or CABG
Anti-arrhythmics other than ß-blockers and Ca channel blockers
(2 excluded for noise/ artifact)) |
Not with-held
96.5% during ICU stay |
Yes, EF ≤40% 31.8%
∑Mean 45.3%
TWA >46 uV
Channel 1 All 8.5%
Channel 1 ↓ EF 11.1%
Channel 2 All 6%
Channel 2 #8595; EF 6.3% |
No
18.7% |
| Exner 2007
REFINE
Multi-center Canada
See Spectral. |
MI
EF <40% w/in 48o of MI
EF <50% beyond 48o of MI
ICD implantation permitted (n=16) |
A fib
Incapable of exercise test
ICD, pacemaker
↓ life expectancy |
Dose prior to test delayed
Use reported
92% |
No
EF 40% if CD/arrest
Vs 49% w/o when measured at 8 wks |
No
38% vs 22% if cardiac death/
arrest |
Slawnych 2009*
REFINE
Only p exercise data for MMA |
See above |
See above |
See above |
EF 38% if CD vs 48% w/o when measured at 8 wks |
No
35% vs 21% if cardiac death |
| Hoshida 2013 |
MI >2 wks prior
NYHA I-II |
Atrial fib, pacemaker, ♥ resync tx, intraventricular block
Could have ICD |
Use reported |
No (mean EF 47%) |
No |
| Yu 2012 |
MI w/in 15 days
P percutaneous coronary intervention PCI
EF <50% |
Atrial fib/flutter, AV block, pacemaker, sinus brady-cardia, ICD, EF <20%, s/p CABG, age ≥ 80 |
Use reported |
EF >35%, ≤35% |
No |
| Nieminen 2013
MERLIN-TIMI subset
Multi-center International |
MI wo ST segment elevation & w/in 48o of last ischemic sx
TIMI ≥3
For substudy
EF<40%
VT in hospital or SCD |
Persistent ischemia
Rx ↑QT interval
Liver dx, dialysis
↓ life expectancy |
|
No
HF 36% |
No
All 42%
+SCD/VT 36%
-SCD/VT 48% |
Li-na 2012 |
MI w/in 1-3 wks ± DM
(All: Holter being done) |
Atrial fib/flutter, AV block, BB block, pacemaker, PVCs, renal or liver disease |
Not with-held
MI+DM 78%
MI w/o DM 77%
No CAD 9% |
No
MI+DM EF45%
MI w/ DM EF 46%
No CAD EF 59% |
Yes |
| Stein 2008
EPHESUS subset
Multi-center International |
MI w/in 2 wks
EF ≤40% early p MI & w sx unless w DM |
K-sparing diuretic
↑K, creatinine >2.5 mg |
Use reported
SCD 14 (78%)
OCD 21 (75%)
Alive 63 (68%) |
No
EF
SCD 33±6%
OCD 31±6%
Alive 34±5% |
No
+ DM
SCD 38%
OCD 36%
Alive 33% |
| Sulimov 2012 |
MI last 2 mo |
Atrial fib/flutter, AV block, pacemaker, anemia, hyperthyroidism, CA, certain rx |
Use reported |
EF 40% ? post hoc |
No |
| ↓ Left Ventricular Function |
| Sakaki 2009
(mixed ischemic & non-ischemic) |
Referral for 24 hour ECG |
Paroxysmal atrial fibrillation SVT, and extra systoles |
Use reported (73%)
TWA+ (77%)
TWA- (72%) |
No
EF 34%
NYHA Class III and IV 14% |
No
Dead 52%
Alive 24% |
| Heart Failure |
| Kim 2014
1 center US |
ICU w new HF or acute decompensation
ICD, CRT permitted |
Congenital heart disease, pericardial effusion, valve disease |
Use reported
TWA+ MA+ 73.1%
TWA+ MA- 60%
TWA MA – 68.1% |
No |
No |
| Exercise Test Referral |
| Leino 2011
FINCAVAS
1 center Finland |
Referred for exercise testing |
A fib |
Use of ß-Blocker or withdrawal <48o bf test
Died 172 (74.5%)
Alive 1656 (49.3%) |
No |
No
Died 17%
Alive 10.9% |
Minnikinen 2009
Earlier subset of above |
Referred for exercise testing |
|
Use reported
59% |
No |
No
12% |
Leino 2009
Earlier subset of above |
Referred for exercise testing |
A fib excluded for heart rate recovery testing |
Use of ß-Blocker
Died 99 (78%)
Alive 1174 (57.3%) |
No
LVfxn assessed 55% |
No
Died 18.1%
Alive 11.6% |
Nieminen 2007
Earlier subset of above |
Referred for exercise testing
A fib not excluded |
|
Use reported
TWA ≥65 µV 62%
TWA <65 µV 65% |
No
LVfxn assessed 51%
NYHA Class III
TWA ≥65 µV 11%
TWA <65 µV 10% |
No
≥65 µV 25%
<65 µV 16% |
Slawnych 2009
Subset of Nieminen
2007
Part of a paper combining REFINE & FINCAVAS) |
Referred for exercise testing
Limited to Nieminen 2007 study pts w CVD |
|
Use reported
+CD 74%
Alive/OD 94% |
No
Median EF for391 of 681 47% |
No
+CD 29%
Alive/OD 18% |
| Angiography Referral |
| Kenttä 2014
ARTEMIS substudy |
CAD±T2DM |
A fib, BBB
NYHA III/IV, EF <40%
Unstable angina, HF, PAD,
Retinopathy, Neuropathy
>75 yrs, BMI >40 kg/m2 |
Use reported
CAD+DM
+Exercise 30 (100%)
-Exercise 27 (90%)
CADnoDM
+Exercise 28 (80%)
-Exercise 32 (91%) |
No
EF: CAD+DM
+Exercise 66.2%
-Exercise 65.5%
EF: CADnoDM
+Exercise 66.6%
-Exercise 64.8% |
Yes |
| Hypertension |
| Surgit 2014a
1 center Turkey |
Hypertension |
CAD, CHF, COPD, valve dx, heart block, liver dx, renal dx, ß-clocker or Ca channel blocker use |
None |
No |
No
Normal 19%
Concentric remodeling 24% Concentric hypertrophy 26%
Eccentric hypertrophy 25% |
| Surgit 2014b
1 center Turkey |
Hypertension |
CAD, CHF, COPD, valve dx, heart block, liver dx, renal dx, ß-clocker or Ca channle blocker use |
None |
No |
No
Control 11%
Dipper 16%
Non-dipper 19% |
| Ambulatory Elderly |
Stein 2010
Cardiovascular Health Study subset |
SCD if event did not occur in hospital or nursing home to ↓ co-morbidity |
- |
Use reported & controlled |
- |
Matched |
Table 1C Studies Using Modified Moving Average Method
| Post Myocardial Infarction (MI) |
Test Vehicle |
Leads |
MMA Cutpoint (uV) |
Update Factor |
When |
Duration |
| Arisha 2013 |
Holter while hospitalized for MI, ECHO |
? V1, V2 |
>46 µV |
|
While hospitalized |
≤6 mo.
Mean not reported |
| Exner 2007
REFINE
Multi-center Canada
See Spectral |
Exercise/recovery ECG, SAECG, amb Holter , baroreflex sensitivity using phenylephrine, LVfxn |
- |
MMA cutpoint based on ROC data ≥5 µV
Spectral ? |
32 beat window or 1/16 for MMA |
Initial tests 2-4 wks p MI
Repeat tests 10-14 wks p MI |
Median 3.7 yrs |
| Slawnych 2009*
REFINE
Only p exercise data
for MMA |
Recovery ECG |
V1, V2, Z |
See above
MMA cutpoint based on ROC data ≥20 µV & ≥60 µV |
See above
1/8 to 1/64 |
See above
Repeat tests done at 10-14 wks p MI |
Median 3.9 yrs |
| Hoshida 2013 |
Amb Holter, ECHO |
V1, V5 |
>64 µV |
1/8 |
>2 wks p MI |
3.2 yrs |
| Yu 2012 |
Amb Holter, ECHO |
V2, V5 |
≥47 µV |
1/8 |
W/in 15 days |
1.3 yrs |
| Nieminen 2013
MERLIN-TIMI subset
Multi-center International |
Amb Holter, LCfxn |
V1, V5, AVF |
≥47 µV |
|
W/in 2 days p MI |
~1 yr not stated for group |
| Li-na 2012 |
Amb Holter, ECHO |
V1, V3, V5 |
≥47 µV |
1/8 |
W/in 1-3 wks |
1.6 yrs |
| Stein 2008
EPHESUS subset
Multi—center International |
Amb Holter, LVfxn |
V1, modified V3, other lead often missing |
≥47 µV
Also looked for best cut-point w these data: V1 ≥43 µV
V3 ≥47 µV |
1/8 |
W/in 14 days |
~1.4 yrs |
| Sulimov 2012 |
Amb Holter, ECHO |
below ~V1, V2 |
Determined during study
>53 µV 100 bpm >18 µV 5 AM |
1/8, 1/32 |
2 mo p MI |
1 yr |
| ↓ Left Ventricular Function |
| Sakaki 2009
(mixed ischemic & non-ischemic) |
Amb Holter, ECHO |
V1, V5 |
≤ 65mV |
1/8 |
While hospitalized |
1.3 yrs |
| Heart Failure |
| Kim 2014
1 center US |
Amb ECG, Amb BP, ECHO |
|
≥47 µV |
1/8, 1/32 |
NA |
Median 0.8 mo |
| Exercise Test Referral |
| Leino 2011
FINCAVAS |
Rest/exercise ECG
(unclear which used) |
V1-V6 w selection of V5 based on results |
≥46 µV |
1/8 |
NA |
4.2 yrs |
Minnikinen 2009
Earlier subset of above |
Rest/exercise/recovery ECG
(unclear which used) |
V1-V6 |
Calculated continuously
5, 10, 20, 30, 40, 50, 60, 70, 90, 100, 110, 120 µV tested |
1/8 |
NA |
3.9 yrs |
Leino 2009 ex recovery
Earlier subset of above |
Rest/exercise ECG
(unclear which used) |
V1-V6 |
Calculated continuously
20 µV & 60 µV used |
1/8 |
NA |
3.9 yrs |
Nieminen 2007
Earlier subset of above |
Rest/exercise/recovery ECG
(unclear which used) |
12 lead ECG |
Calculated continuously
46, 50, 60, 65, 70 µV tested
46 µV & 65 µV used |
1/8, 1/32 |
NA |
3.6 yrs |
Slawnych 2009
Subset of Nieminen
2007
Part of a paper combining REFINE & FINCAVAS |
Recovery ECG |
12 lead ECG w V1, V5, Z (AVF) used to compare to REFINE |
20 µV & 60 µV used |
1/8 |
NA |
3.9 yrs |
| Angiography Referral |
| Kenttä 2014
ARTEMIS substudy |
Exercise ECG, LVfxn |
|
≥47 µV |
|
NA |
~1 yr not stated for group |
| Hypertension |
| Surgit 2014a
1 center Turkey |
Exercise ECG, ambulatory blood pressure monitoring, transthoracic ECHO. |
12 lead |
≥65 µV |
1/8 |
NA |
NA |
| Surgit 2014b
1 center Turkey |
Exercise ECG, ambulatory blood pressure monitoring, transthoracic ECHO. |
12 lead |
≥65 µV |
1/8 |
NA |
NA |
| Ambulatory Elderly |
| Stein 2010
Cardiovascular Health Study subset |
Amb Holter |
Believed to be V5, AVR |
Exploratory for this patient population
37 µV AVR |
- |
Part of survey |
Follow-up ≤13 yrs |
Table 1D Studies Using Modified Moving Average Method
| Post Myocardial Infarction (MI) |
Consort |
N |
Age |
# + MMA |
Indeterminate + |
Events SCD +other cardiac+non-cardiac |
| Arisha 2013 |
No |
199 |
61.7±14.8 |
Channel 1 All 8.5%
Channel 2 All 6% |
|
SCD 3+ External cardioversion 2 + 1 cardiogenic shock 4 + 1 wall rupture + sepsis 1 |
| Exner 2007
REFINE
Multi-center Canada
See Spectral. |
Yes
5599 screened |
322 tested
Not eligible:
EF preserved 3040, A fib/pace-maker 296, ICD 89, Incapable of exercise test 338,
Geo isolation 712,
↓ life expectancy 472 |
Median 62 |
Not stated
TWA method concordance K=0.17
Concordance ↑ in pts w events |
Non-neg used |
Resusc arrest including 2 in pts an ICD + 30 CD |
Slawnych 2009*
REFINE
Only p exercise data for MMA |
See above |
See above |
Median 62 |
Not stated |
Not stated, presumably as above |
See above
17 SCD + 3 0CD + OD |
| Hoshida 2013 |
No |
313 |
70±12 |
14
(HRT + 61) |
Not clearly stated |
(6 SCD+6 Fatal Arrthythmia including 1 on ICD)+ 16 OC (HF) +3 NC |
| Yu 2012 |
No |
227 |
CALC |
48 |
Not clearly stated |
10 SCD+1 OC+1 NC
18 EF≤35 (4 SCD) |
| Nieminen 2013
MERLIN-TIMI (ranolazine vs placebo) subset |
No
6560 ran-domized |
210
101 SCD or VT ≥4 b
(SCD 16)
109 w/o above |
68 (58-75) |
+ 40 (19%) |
|
SCD/VT |
| Li-na 2012 |
No |
248
MI+DM 77
MI w/o DM 96
W/o CAD 75 |
MI+DM 66
MI w/o DM 65
W/o CAD 62 |
MI+DM 46
MI w/o DM 51
W/o CAD 4 |
Not clearly stated |
3 SCD + 7 OCD + (2 OD excluded)
MI+DM 1 SCD = 6 OCD
MIw/oDM 2SCD + 4 OCD |
| Stein 2008
EPHESUS subset
Multi-center International |
No
6632 ran-domized |
493 w Holter data
46 died
92 matched controls |
SCD 68±11
OCD 66±11
Alive 66±11 |
Mean V1
SCD 38±12
OCD 26±8
Alive 29±11
Mean V1
SCD 45±19
OCD 33±13
Alive 33±25 |
|
Mean V1
SCD 38±12
OCD 26±8
Alive 29±11
Mean V1
SCD 45±19
OCD 33±13
Alive 33±25 |
| Sulimov 2012 |
No |
111 MI
60 Control |
64.1±10.5 |
Depends on cut-point & time of day |
Maximal values & not a predetermined cut-point used |
15 SCD + 5 OCD +3 OD |
| Left Ventricular Function |
| Sakaki 2009
(mixed ischemic & non-ischemic) |
No |
312 enrolled
17 excluded |
66 ± 16
Died 74 ± 12
Alive 65 ± 16 |
12% ischemic
29% non-ischemic |
Not stated |
5 SCD + 9 CHF death + 12 unspecified cardiac death + 10 OD + 1 ICD discharge |
| Heart Failure |
| Kim 2014
1 center US |
No |
133 enrolled
22 excluded (balloon pump, CP support, a fib, PVCs) |
TWA+MA+ 62.3
TWA+MA- 63
TWA-MA- 57.6 |
TWA+MA+ 25%
TWA+MA- 4%
TWA-MA- 71% |
Unclear |
42 died + 2 transplant |
| Exercise Test Referral |
| Leino 2011
FINCAVAS |
No |
3598 |
55.6+12.9 yrs
Died 63.7±11.4
Alive 55.1±12.8 |
Reported data by quin-tiles & not by ≥46 µV cutpoint |
|
46 SCD + 51 OCD + 134 OD |
Minnikinen 2009
Earlier subset of above |
No |
2119 |
57.4 |
Depends on selected cut-point. ~40 µV found to be important |
|
33 SCD + 31 OCD + 64 OD |
Leino 2009ex recovery
Earlier subset of above |
No |
1972 (excluding 31 w a fib that interferes w heart rate recovery assessment) |
Died 65.1±11.3
Alive 56.5±13.1 |
Exercise 5%
Recovery ≤ 20µV 51%
≤ 60 µV 4% |
|
NR SCD + 55 CD + 61 OD |
Nieminen 2007
Earlier subset of above |
No |
1037 |
58±13
+TWA 60±12
-TWA 58±13
(cut-point ≥65) |
TWA ≥65 87
TWA<65 950 |
|
20 SCD + 14 OCD + 4 unk + 21 OD |
Slawnych 2009
Subset of Nieminen
2007
Part of a paper combining REFINE & FINCAVAS |
No |
681 of the above |
Median
CD 69
Alive/OD 62 |
Not stated |
|
NR SCD + 34 CD + OD |
| Angiography Referral |
| Kenttä 2014
ARTEMIS substudy |
No
Randomized
exercise subset 224
Final + 130 including matched controls used |
Not ITT: Additional exclusions:
CAD+DM
+Exercise 30
-Exercise 30
CADnoDM
+Exercise 35
-Exercise 35 |
CAD+DM
+Exercise 61.7
-Exercise 61
CADnoDM
+Exercise 61.3
-Exercise 60.7 |
+ 93 (72%) |
Unclear |
No deaths; some changes in TWA status |
| Hypertension |
| Surgit 2014a
1 center Turkey |
No |
N=311
Normal geometry 90
Conc remodeling 99
Conc hypertrophy 63
Eccentric hypertrophy 58 |
Normal geometry 49.6
Conc remodeling 50.9
Conc hyper-trophy 51.6
Eccentric hyper-trophy 51.6 |
Normal geometry + 12.6%
Conc remodeling + 16.2%
Conc hyperrophy + 39.7%
Eccentric hyper-trophy + 37.3% |
Unclear as to whether indeter-minate values were used |
NA |
| Surgit 2014b
1 center Turkey |
No |
N=118
Normal 63
Dipper HTN 61
Non-dipper HTN 57 |
Normal 46
Dipper HTN 46
Non-dipper HTN 48 |
Normal 4%
Dipper HTN 15%
Non-dipper HTN 27% |
Unclear as to whether indeter-minate values were used |
NA |
| Ambulatory Elderly |
| Stein 2010
Cardiovascular Health Study subset |
No |
49 SCD
97 Control |
73±5 |
Study limited to those with SCD & matched controls |
Various cut points used |
49 of 1649 with usable Holters died of SCD over up to 14 yrs |
Figure 1. Testing for micro T-wave alternans (also termed repolarization alternans) attempts to identify variations in the vector and amplitude of the T wave component of the ECG. Because the amount of variation is minute and not visible to the eye, sensitive digital signal processing techniques are required.
Panel A. Heart beats are aligned using the QRS element of the ECG. Panel B. The beat-to-beat fluctuation in amplitude (oscillation) of the T-wave reflect the “alternans” at each timepoint. Panel C. In Spectral Analysis, a fast Fourier transformation of the electrical signal generates a power spectrum. The “alternans” is the peak at the frequency equaling half of the heart rate. Panel D. In the Modified Moving Average, the signals from heart beats are distributed into series of even and odd beats and then a non-linear filter is used to reduce noise and quantify the maximum difference between the even and odd beats. (Figure from Cox 2007.)