To: Administrative File CAG00066R-2
Ocular Photodynamic Therapy (OPT) with Verteporfin
From: Sean Tunis, MD, MSc
Director, Coverage and Analysis Group (CAG)
Jeffrey Shuren, MD, JD
Division Director, Division of Items and Devices
Ronald Dei Cas, MD
Medical Officer, CAG
Poppy Kendall, MHS
Policy Analyst, CAG
Samantha Richardson
Policy Analyst, CAG
Subject: Coverage Decision Memorandum for Ocular Photodynamic Therapy with Verteporfin
Date: March 28, 2002
This memorandum serves five purposes: (1) describes the pathophysiology of age-related macular degeneration (AMD); (2) discusses available treatments and recent developments in the clinical management of AMD; (3) summarizes the relevant clinical literature on the use of ocular photodynamic therapy (OPT) with verteporfin; (4) explains our reasons for issuing a reconsideration of the October 17, 2001 coverage decision memorandum; and (5) announces our intent to retain the current national noncoverage of OPT with verteporfin for AMD patients with subfoveal occult with no classic choroidal neovascularization (CNV).
I. Clinical Background
Age-related macular degeneration is the leading cause of legal blindness in Americans over the age of 65. The estimated prevalence of AMD in Americans 75 years of age or older is 7.1%. 1 While the exact etiology of AMD is not well understood, it is thought to be a multi-factorial disease. In addition to age, several other risk factors are associated with AMD. These include family history of AMD, smoking, and light eye color. Recent findings also suggest that low dietary intake of antioxidants may predispose people to AMD.
AMD involves the destruction of normal macular function. In AMD, acellular debris called drusen accumulates within Bruch's membrane. Bruch's membrane, as shown in Figure 1, is the layer between the outer edge of the retina and the choroid. This layer is important because it keeps the blood vessels of the choroid from leaking fluid into the retina.
Figure 1
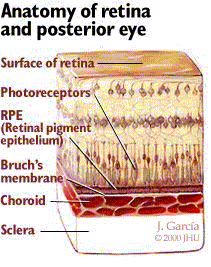 There are two basic types of AMD: dry and wet. Dry AMD is the most common type, accounting for 90% of all cases. In dry AMD, the accumulation of drusen, and the resulting effect they have on macular function, leads to central vision deterioration. Wet AMD accounts for 10% of cases, and poses a higher risk of severe central vision loss. In wet AMD, breaks in Bruch's membrane allow vessels from the choroid to grow, leak, and bleed into the subretinal space; this is termed choroidal neovascularization (CNV). CNV can cause large distortions of the macula, and can progress quickly (over the course of days or weeks), effectively destroying central vision. While AMD is the most common condition associated with CNV, other retinal disorders such as pathologic myopia, presumed ocular histoplasmosis syndrome, angioid streaks, and retinal hamartomas can be complicated by CNV formation. There is no definitive treatment for dry AMD. For patients with wet AMD, laser photocoagulation has been shown to help reduce the rate of vision loss in some patients.
There are two basic types of AMD: dry and wet. Dry AMD is the most common type, accounting for 90% of all cases. In dry AMD, the accumulation of drusen, and the resulting effect they have on macular function, leads to central vision deterioration. Wet AMD accounts for 10% of cases, and poses a higher risk of severe central vision loss. In wet AMD, breaks in Bruch's membrane allow vessels from the choroid to grow, leak, and bleed into the subretinal space; this is termed choroidal neovascularization (CNV). CNV can cause large distortions of the macula, and can progress quickly (over the course of days or weeks), effectively destroying central vision. While AMD is the most common condition associated with CNV, other retinal disorders such as pathologic myopia, presumed ocular histoplasmosis syndrome, angioid streaks, and retinal hamartomas can be complicated by CNV formation. There is no definitive treatment for dry AMD. For patients with wet AMD, laser photocoagulation has been shown to help reduce the rate of vision loss in some patients.
Patients suspected of having wet AMD generally undergo fluorescein angiography. There are two basic patterns of fluorescein leakage in wet AMD: classic and occult. In pure classic CNV, the choriocapillaris plexuses that are involved can be seen distinctly. In pure occult lesions, the location of the offending vessels responsible for the leakage is not recognizable. Many CNV lesions are a combination of both occult and classic with a portion showing a defined site of leakage and another portion being obscured. CNV in AMD is further characterized by one of three locations: subfoveal, juxtafoveal and extrafoveal. Subfoveal, as the name implies, is CNV that lies directly below the fovea. Juxtafoveal and extrafoveal CNV lie progressively further away from the fovea (but still within the macula).
Laser photocoagulation has been shown to decrease vision loss by 50% in juxtafoveal and extrafoveal CNV. For subfoveal CNV, laser treatment has been shown to have some benefit, mainly in patients with classic CNV. Laser photocoagulation by itself destroys the retina overlying its area of application. When applied away from the foveal center (i.e., juxtafoveal or extrafoveal) the effect of the laser itself on vision is variable. When applied to the foveal center, as in cases of subfoveal CNV, the laser is almost assured to destroy some central vision. In addition, subfoveal CNV recurs approximately 50% of the time after "successful" laser therapy. Thus, while laser photocoagulation of subfoveal CNV theoretically maybe preferable to allowing the disease to progress naturally, that possible benefit carries potential risks.
Ocular Photodynamic Therapy (OPT) with Verteporfin: OPT for the treatment of CNV involves the intravenous injection of a photosensitive drug, verteporfin. A laser, which emits light only at verteporfin's absorption peak of 689 nm, is then directed into the eye. It is thought that the excitation of verteporfin generates singlet oxygen and other reactive intermediates that result in temporary closure of leaking blood vessels. The laser is non-thermal; thus it does not produce a heat effect on the retina and causes no damage to the retinal tissue. Verteporfin therapy is neither a cure nor a preventative for CNV in AMD; it is meant to slow progression of the disease. Indeed, its effect is generally not permanent. The closure of leaking blood vessels caused by OPT is often temporary, and these vessels may re-open. Additional OPT treatments, therefore, may be needed.
II. History of Medicare's Coverage on OPT with Verteporfin and Timeline of Recent Activities
Food and Drug Administration (FDA) Status: On April 12, 2000, the Food and Drug Administration (FDA) approved the use of verteporfin in AMD-related subfoveal CNV in which >50% of the lesion is classic (i.e. predominantly classic) as determined on fluorescein angiogram. On August 22, 2001, the FDA approved verteporfin for the treatment of predominantly classic subfoveal CNV related to pathologic myopia as well as ocular histoplasmosis. The use of OPT with verteporfin for subfoveal occult with no classic CNV in AMD is an off-label use.
Current Medicare Policy of OPT with Verteporfin: On November 8, 2000, the Centers for Medicare and Medicaid Services (CMS) issued a coverage Decision Memorandum on the use of OPT with verteporfin for the treatment of AMD. In this memorandum, CMS announced its intent to cover OPT with verteporfin for AMD-related subfoveal CNV in which >50% of the lesion was classic on fluorescein angiogram; other forms of AMD would be non-covered. This decision was based on data from the Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) Study Group, a multi-center, randomized controlled clinical trial. Prior to this November 2000 decision memorandum, CMS was silent on the use of OPT with verteporfin in AMD. Coverage instructions for this policy were issued on February 26, 2001(CIM §35-100 and § 45-30), and became effective on July 1, 2001. On May 21, 2001, the Vitreous Society submitted a formal request for reconsideration of coverage for OPT with verteporfin in the treatment of AMD with occult but no classic CNV. On October 17, 2001, CMS issued a national coverage decision memorandum which described our intent to cover OPT with verteporfin for AMD patients with occult with no classic CNV. Coverage instructions for the policy announced in this decision memorandum, which was based on the results published in the Verteporfin in Photodynamic Therapy (VIP) trial,2 have not been issued.
Recent Activities: Soon after we posted the October 17, 2001 decision memorandum on our website, experts in the field, both within and outside the federal government, raised critical new questions concerning the underlying data from the clinical trial upon which CMS based its decision. Specifically, questions were raised about the decision, conduct, and reporting of the study. CMS was concerned that in relying solely on the published results of a single study, and especially given that our conclusions were in part based upon subgroup analyses, we may have materially misinterpreted data crucial to determining the effectiveness of this treatment. We believed that further review was needed to fully understand theses concerns, and we decided to undertake a reconsideration of our October 17, 2001 decision memorandum consistent with the process described in our April 27, 1999 Federal Register Notice (64 FR 22619). This reconsideration, as announced on October 29, 2001, consisted of a re-review of the VIP study. We asked the requestor and the manufacturer of verteporfin to provide us with the data underlying the VIP study that was not presented in the published report, as well as additional analyses of data presented in that report. Several public and private organizations, such as the FDA, the National Eye Institute, the Vitreous Society, and the manufacturer, were consulted. Our principal concerns centered around two issues: the net clinical benefit this treatment might provide patients who have occult with no classic CNV, and the confidence we had that the observed effect reflected the true effect. In the course of our reconsideration, we also raised questions of how patient attrition was handled in the data analysis, and whether the delayed benefit was due in part to occult lesions converting to classic lesions.
III. Summary of Evidence
Medical Literature
CMS's analysis of the scientific evidence supporting the use of OPT with verteporfin in the aforementioned group of patients concentrated primarily on one study - the Verteporfin in Photodynamic Therapy Study Group (VIP) trial. Reports from two prior studies, The Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group (TAP) reports 1 and 2 were also consulted.3,4 The TAP 1 and 2 reports formed the basis of the national coverage determination on OPT with verteporfin which was issued in November 2000. The TAP trial preceded the VIP trial, and the two studies had similar designs and outcome measures.5 The TAP trial primarily looked at patients in whom the CNV was classic in nature. A brief summary of the VIP study follows.6
The VIP study was a double-masked, placebo-controlled, randomized, multi-center clinical trial involving 339 patients from 28 clinical centers in North America and Europe. The study's purpose was to determine if OPT with verteporfin could safely reduce the rate of vision loss in patients with AMD-associated subfoveal CNV that were not included in the TAP trial. The VIP study enrolled patients who had: (1) occult but no classic CNV on fluorescein angiogram; or (2) presumed early-onset classic CNV with baseline visual acuity of 20/40 or better. The study was powered for these two groups combined. Inclusion criteria for the VIP study (including both subgroups listed above) were:
- subfoveal CNV;
- area of the CNV at least 50% of the area of the total neovascular lesion;
- greatest linear dimension ≤ 5400 microns;
- presumed recent progression of disease;
- best-corrected visual acuity at baseline of 20/100 or better; and
- ability and willingness to provide written informed consent.
Patients were randomized to either the verteporfin treatment group or the placebo group. Patients in the verteporfin group received an intravenous injection of 6 mg/m2 body surface area of verteporfin in 30 cc of 5% Dextrose over 10 minutes. The placebo group received 30 cc of 5% Dextrose intravenously over 10 minutes. Fifteen minutes after the start of the infusion, the patient's enrolled eye was exposed to a 689 nm wavelength nonthermal laser for 83 seconds. All patients were scheduled for regular three-month follow-up visits. At each regularly scheduled exam, the patients' vision was tested, a dilated fundus exam was performed, and a fluorescein angiogram done. Physicians assessing the patients at each follow-up were masked to the treatment group. If there was any leakage seen on the fluorescein angiogram, the patient was retreated with the same agent to which they were randomized. Patients were followed for 24 months. To account for missing data points a "last observation carried forward" approach was used in the data analysis. To preserve randomization, guard against patient selection bias, and account for crossovers in the study groups, the authors employed an "intent-to-treat" analysis. The treatment protocol was maintained throughout the 24 months, and masking was preserved throughout the two year time period.
A total of 339 eyes in 339 patients were included in this study. 258 eyes (76%) had occult with no classic CNV and 81 (24%) had early onset classic with good vision. The study's primary endpoint was the percentage of eyes that suffered moderate vision loss, as compared to baseline, at the 12-month follow-up. Moderate vision loss was defined as loss of 15 or more letters on a standardized eye chart. This corresponds to a loss of approximately three lines of vision from a standard Snellen eye chart. Of the 258 eyes in the occult with no classic subgroup, 166 eyes received treatment with verteporfin and 92 eyes received treatment with placebo. Of the 81 eyes with early classic and good vision CNV, 59 were treated with verteporfin and 22 were in the placebo group. In addition to moderate vision loss at 12 months, the authors reported the results of moderate vision loss at 24 months for the entire study population. They also presented data on severe vision loss (loss of 30 or more letters, approximately 6 lines of vision, from baseline) for the entire study population at 24 months.
As stated above, the study's primary endpoint was moderate vision loss at 12 months. For the entire study group, the results did not achieve statistical significance at the primary endpoint (see Table 1). By 24 months, the entire study group of all eyes treated with verteporfin did show a benefit in terms of moderate vision loss (Table 1). The authors presented data on severe vision loss for the entire study group only for the 24-month point; these results were significant.
TABLE 1: Moderate and Severe Vision Loss at 12 and 24 month Follow-Up, All Study patients (occult with no classic and early onset classic with good vision subgroups)
| |
12 month Follow-up |
24 month Follow-up |
|---|
| |
Moderate loss |
Severe loss |
Moderate loss |
Severe loss |
| Verteporfin |
51% (114/225) |
no data |
54% (121/225) |
30% (67/225) |
| Placebo |
54% (62/114) |
no data |
67% (76/114) |
47% (54/114) |
| |
P=0.52 |
no data |
P=0.023 |
P=0.001 |
The authors performed several subgroup analyses. As Table 2 shows, the subgroup of occult with no classic CNV did not show a statistical benefit in terms of verteporfin treatment at the study's primary endpoint. In addition, there was no statistically significant benefit for verteporfin treatment in terms of severe vision loss at 12 months. Treatment with the drug did reach statistical significance for both moderate and severe vision loss at 24 months.
TABLE 2: Moderate and Severe Vision Loss at 12 and 24 month Follow-Up, Occult with no Classic Subgroup
| |
12 month Follow-up |
24 month Follow-up |
| |
Moderate loss |
Severe loss |
Moderate loss |
Severe loss |
Verteporfin |
51% (84/166) |
22% (36/166) |
55% (91/166) |
29% (48/166) |
| Placebo |
55% (51/92) |
33% (30/92) |
68% (63/92) |
47% (43/92) |
| |
P=0.51 |
P=0.07 |
P=0.032 |
P=0.004 |
Mild adverse events, thought to be related to treatment, were noted in 43% (96/225) of the verteporfin group (both occult with no classic and early onset classic) and 18% (21/114) of the placebo group. These adverse events consisted of slight visual disturbances unsubstantiated on exam, injection site related problems, infusion related back pain, allergic reactions, and photosensitivity. None of these events were considered to be serious.
A more serious adverse event, severe vision loss, was noted in ten of the 225 verteporfin-treated patients (4.4%) within seven days of treatment. None of the placebo patients experienced severe vision loss. This vision loss was characterized as at least a 20 letter decrease in visual acuity as compared to pretreatment acuity. Eight of these 10 patients had occult with no classic CNV at baseline. Thus, severe vision loss was seen in 4.8% (8/166) of the occult with no classic subgroup. The authors stated that by three months after this event, five of the 10 patients recovered vision to less than 20 letters lost as compared to their pretreatment acuity. According to the study investigators, some of these five patients who regained vision were in the occult with no classic group. It should be noted that severe vision loss in the TAP study (upon which we based our coverage of predominantly classic CNV in AMD) was noted in only 1% of verteporfin-treated patients. The reason for this markedly elevated risk of severe vision loss in the VIP study is unclear.
As stated earlier, information brought to our attention following the release of our October 17, 2001 coverage decision memorandum made us question our original interpretation of the data. Broadly stated, we had two major and two minor concerns. To better understand these issues, and reach a decision that was in accord with the science, we went back to the requestor and the manufacturer of verteporfin and asked for data from the study that was not presented in the published report. The two major issues that concerned us were what was the observed effect and could we be confident that the observed effect reflected the true effect of treatment with verteporfin. Two other issues that we raised with the requestor were the use of "last observation carried forward" to account for patient attrition, and whether the delayed benefit was due in whole, or in part, to occult CNV converting to classic CNV.
From Tables 1 and 2, we see that OPT with verteporfin (in either subgroup) did not reach statistical significance for preventing moderate or severe vision loss at 12 months. The treatment did reach statistical significance for both measurements of vision loss at 24 months, however, the difference was only mildly significant for moderate vision loss. One limitation of making a decision based solely on the results reported in Tables 1 and 2 is that these numbers only tell us about the sample population at one point in time, 24 months. It does not give insight into vision preservation over the course of the patients' treatments, and what this means in terms of actual vision.
Kaplan Meier survival curves, which were not presented in the published study, are useful in following data over time to a specified endpoint because they do not rely on predetermined intervals for looking at data. Indeed, in Kaplan Meier curves, events can be identified at the exact point in time at which they took place. To better understand the observed course for patients with occult only CNV, we asked that the requestor provide us with several Kaplan Meier curves. Three of the most informative curves were 1) time to ≥ 15-letter loss, 2) time to ≥ 30 letter loss, and 3) time to vision of 20/200 or worse. In each curve, the occult-only patients who received treatment were plotted alongside the placebo group.
Figures 2 and 3 show the curves of time to moderate (≥ 15 letter) and severe (≥ 30 letter) vision loss comparing verteporfin and placebo occult with no classic groups. The time to ≥ 15 letter loss curve shows that the verteporfin and placebo group are essentially parallel until the 9 to 12-month period at which point they diverge slightly. Both continue to decrease at the same rate over the remaining months, and the difference in the curves is not significant (p=0.13). The time to ≥ 30 letter loss shows the curves overlapping until month 12. Afterwards the verteporfin group has a slower rate of visual degradation, and the p value is significant (p=0.022). For each figure, however, vision loss continues in both the verteporfin and placebo groups. It should be noted that these curves include the patients who had severe vision loss within seven days of verteporfin treatment (see above). Again it must be noted that the study's primary endpoint was moderate vision loss at 12 months, and the only statistically significant result seen in the Kaplan Meier curves was for severe vision loss at 24 months. Although the requestor and the manufacturer hypothesized that the population of occult with no classic patients treated with verteporfin may show a benefit later in time than patients with predominantly classic CNV (because the natural history of vision loss in the occult-only patients is a slower decline than in other forms of "wet" AMD), the raw data and the Kaplan Meier curves are not consistent with this theory.
Figure 2

p=0.130
Figure 3
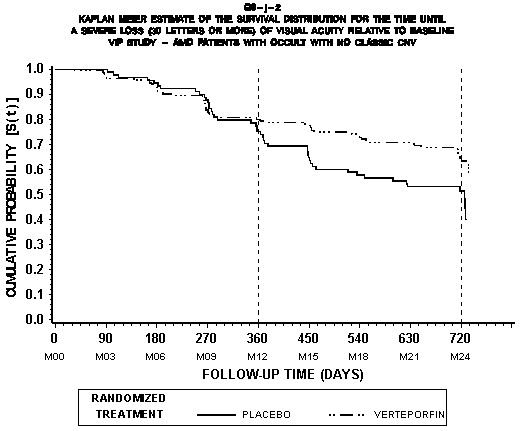
p=0.022
In order to get an idea of what the functional path was for treated and placebo patients, we asked the requestor for the Kaplan Meier curve of the time to 20/200. This was chosen because 20/200 is one threshold used to determine legal blindness. Of course, legal blindness is determined by vision in the better eye, and we are not using this curve to infer time to legal blindness. Nonetheless, it does give an estimate of time until vision reaches a level that is considered by many to be sufficiently poor so as to significantly affect activities of daily living. As Figure 4 shows, the curves were essentially the same until the 9-month point at which time the placebo-treated patients showed a faster rate of progression to 20/200. The difference between the curves was statistically significant (p=0.02). In terms of days of delay in decline of vision, the median number of days to 20/200 vision was 734 for the verteporfin-treated group and 617 days for the placebo-treated patients. This is a difference in the median number of days until 20/200 of 117 days. It should also be noted that there are no data on follow-up longer than 24 months. It is not clear that treated patients will experience a long-term stabilization of their vision. A critical issue for CMS was whether we could be confident that the observed effect of 117 days delayed decline in vision loss was real.
Figure 4:
Kaplan-Meier Estimate of the Time Until a Decrease in Visual Acuity to Worse than 34 Letters (Approximately 20/200) in the Treated Eyes for Patients with Occult with No Classic CNV
p=0.02
The requestor provided us with data from the VIP study on moderate vision loss for the treatment and control groups including the actual patient numbers, confidence intervals and significance values. These data, shown in Table 3, are the numbers associated with a bar graph in the published study (see figure 2, page 548 of the VIP study).7 In Table 3 the confidence intervals, which are a way to estimate what is the true effect of treatment, include zero up to and including the month-15 visit and any differences between treatment and placebo groups at such points cannot be considered significantly different. Thus, we cannot be confident that the true effect of OPT with verteporfin, as opposed to the observed effect, is clinically significant. Indeed, there may not be any benefit from treatment at those times. The confidence intervals do not include zero for the month 18, 21 and 24 visits. However, the intervals are fairly wide, and this wideness reduces our confidence that the observed effect of 117 days delayed vision loss is the true effect. Interestingly, the lower limit of the confidence interval at the month 24 visit is closer to zero than the lower limit at the 18 and 21 month interval, a closeness that is also reflected in the modest significance level at the 24-month point (p=0.032).
Table 3:
VIP Trial, Occult with No Classic: Patient Respondersa (Intent-to-Treat)
Number (%) of Patients
|
|
| CNV Lesion Subgroup |
Visit |
Verteporfin |
Placebo |
Differenceb
(Percent) |
95% C.I. of
Difference |
P valuec |
|
| Occult with No Classic Lesions |
|
N=166 |
N=92 |
|
|
|
|
Month 3 |
134 (80.7) |
75 (81.5) |
(-0.8) |
[-10.7, 9.1] |
|
|
Month 6 |
109 (65.7) |
56 (60.9) |
(4.8) |
[-7.5, 17.1] |
|
|
Month 9 |
95 (57.2) |
44 (47.8) |
(9.4) |
[-3.3, 22.1] |
|
|
Month 12 |
81 (48.8) |
41 (44.6) |
(4.2) |
[-8.5, 16.9] |
.515 |
|
Month 15 |
81 (48.8) |
39 (42.4) |
(6.4) |
[-6.2, 19.0] |
|
|
Month 18 |
81 (48.8) |
31 (33.7) |
(15.1) |
[2.8, 27.4] |
|
|
Month 21 |
77 (46.4) |
28 (30.4) |
(16.0) |
[3.9, 28.0] |
|
|
Month 24 |
75 (45.2) |
29 (31.5) |
(13.7) |
[1.5, 25.8] |
.032 |
|
a A responder was a patient who had a decrease from baseline of <15 letters in VA. The results presented in this table are presented in VIP Report No. 2 (Figure 2, page 547).
b Proportion of verteporfin responders minus the proportion of placebo responders.
c Chi-square test used to compare the proportion of patient responders for verteporfin treatment versus placebo at Months 12 and 24.
Another concern CMS had was with the "last observation carried forward" (LOCF) approach to account for missing patient data. This method of data analysis assumes that there is no further change in vision from the time the participant was lost to follow-up until the 24-month endpoint. This assumption did not seem valid given the natural history of progressive visual loss with subfoveal CNV, and could bias the results of the study in favor of the treatment group. In the VIP study, the authors stated that the results were also calculated without the LOCF, and the results were similar. We asked them to provide us with this data.
The requestor and the manufacturer told CMS that the Food and Drug Administration asked that the "last observation carried forward" approach be used in accounting for patient attrition for the purpose of regulatory review. When the data were run without LOCF, the results did not significantly change (Table 4). Thus, it in unlikely that the use of LOCF biased the results in favor of the verteporfin group.
TABLE 4: Moderate and Severe Vision Loss at 12 and 24 month Follow-Up Without the Last Observation Carried Forward
| |
12 month Follow-up |
24 month Follow-up |
|---|
| |
Moderate loss |
Severe loss |
Moderate loss |
Severe loss |
| Verteporfin |
47% (74/157) |
77% (121/157) |
45% (64/143) |
71% (102/143) |
| Placebo |
46% (38/83) |
66% (55/83) |
31% (25/81) |
52% (42/81) |
| |
P=0.84 |
P=0.07 |
P=0.04 |
P=0.0035 |
Another issue was that the benefit seen at 24 months might have been due to the lesions converting at some point between randomization and 24 months from occult with no classic to predominantly classic. There was evidence that some of the occult CNV did develop classic features, and, as the TAP studies showed, OPT with verteporfin was effective in treating predominantly classic CNV due to AMD. If the results changed by removing these patients whose initially occult-only disease later became classic, then it seemed possible that the benefit in the occult-only patients was an artifact of those who had evolving fluorescein angiogram findings (i.e., becoming more like the patients who benefited in the TAP study). CMS requested the VIP Study Group reanalyze their data with this concern in mind.
The VIP Study Group only had angiogram results for time zero, 12 months and 24 months. Also, the angiogram reports at 12 and 24 months noted only whether patients had any amount of classic. Of the 166 occult with no classic patients initially randomized to verteporfin, 117 did not develop classic at the 12-month point. Reanalysis of the data demonstrated that the results reported in the VIP article were not dependent on occult only lesions developing classic components (Table 5).
TABLE 5: Moderate and Severe Vision Loss at 24-month Follow-Up
(comparing verteporfin-treated patients who did not develop classic CNV at 12-months with the total group of placebo-treated patients)
| | 24 month Follow-Up |
|---|
| |
Moderate loss |
Severe loss |
| Verteporfin |
56% (65/117) |
80% (94/117) |
| Placebo |
32% (29/92) |
53% (49/92) |
| |
P<0.001 |
P<0.001 |
In addition to the evidence discussed above, we received 19 letters from individuals and organizations supporting a positive national coverage decision for OPT with verteporfin for the treatment of subfoveal occult with no classic CNV associated with AMD. The organizations that expressed support for this treatment were the Vitreous Society, the Alliance for Aging Research, the National Consumers League, the World Institute on Disability, the National Hispanic Council on Aging, the Gray Panthers, the United Seniors Health Council, and the National Association of Community Action Agencies. These letters of support provided no additional data on clinical effectiveness for this therapy. Some of these letters did provide anecdotal clinical experience with the use of OPT with verteporfin in treating occult with no classic CNV. While we factored these letters of support into our consideration, such letters do not warrant the same weight as other evidence.
Position Statements:
The Vitreous Society has taken the position that the use of verteporfin for select patients with purely occult CNV is standard of care. It released this opinion on its web site (www.vitreoussociety.org) on February 23, 2001 (three months prior to publication of the VIP study due to pre-publication release of the study's data to the Society). Upon publication of the article, the Vitreous Society also sent a "Special Clinical Alert" to its 1500+ members alerting them to the VIP study's findings.
The American Academy of Ophthalmology (AAO) published a Preferred Practice Pattern report on AMD in November 2001. The AAO stated that ocular photodynamic therapy with verteporfin should be considered in the treatment of exudative AMD with subfoveal occult with no classic CNV, particularly if the lesion size is less than 4 disc areas or the level of vision is worse than 20/50.
III. CMS Analysis
National coverage determinations (NCDs) are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally under title XVIII of the Social Security Act. § 1869(f)(1)(B). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be "reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member." § 1862(a) (1) (A).
The VIP study was a well-designed double-masked, placebo-controlled, randomized trial. It was a study that investigated OPT with verteporfin in patients with AMD-associated subfoveal CNV that were not included in the TAP trial. The results reported on patients with occult with no classic CNV are a subgroup analysis. The authors stated that they performed the subgroup analyses because the TAP trial, which had not been completed at the time the VIP trial was undertaken, indicated that the treatment effect varied based on the lesion composition. However, it is unclear that there is a pathophysiologic rationale for separating the patients enrolled in this study into the subgroups of occult with no classic and early onset classic with good vision, which calls into question the validity of performing the subgroup analyses in the first place. This concern is emphasized by the unexplained observation of purported benefit in the predominantly classic AMD patients and the patients with occult disease, but not the subgroup with a minimally classic pattern. Additional subgroups analyses, such as on lesion size or vision at baseline, are even further breakdowns of the data that were performed post-hoc.
CMS believes that the initial subgroup breakdown to occult with no classic might not be so problematic if the reported benefit was large, the treatment was generally safe, and the statistical strength and pattern of the findings were quite strong. None of these conditions were met by the VIP study. A subgroup analysis without a very clear biologic foundation requires strong statistical evidence of a substantial effect in the subgroup to convincingly demonstrate that the observed effect is the true effect. While plausible post-hoc biological explanations have been proposed for the different responses to treatment of various patterns of AMD, the absence of a clear rationale or an unequivocally large effect raise substantial uncertainty as to whether the reported benefits of treatment for occult disease are real.
Arguably any benefit is better than no benefit, especially given a condition for which there is no good treatment. This argument might be convincing if we could be confident that the reported benefit is real. However, CMS's analysis of the data concludes that there is little confidence in the finding of a clinically significant benefit. The authors found that significant differences in moderate and severe vision loss were only noted at the 24-month follow-up. This, however, only gives us data on outcomes at one point in time. To better understand how patients responded to the treatment over the course of the 24 months, we requested Kaplan Meier curves for time to moderate and severe vision loss. The Kaplan Meier curve for moderate vision loss showed no significant difference between the verteporfin and placebo groups (see Figure 2), and the study, therefore, failed to find a benefit for its originally stated primary outcome measure (moderate vision loss at 12 months). The curve for time to severe vision loss was exactly the same for the two groups for the first 12 months (see Figure 3). The verteporfin group then showed a delayed time to severe vision loss at 24 months, although the difference was only modestly significant (p=0.022). The Kaplan Meier curve for time to 20/200 vision showed that OPT with verteporfin differed from placebo by a median of 117 days (see Figure 4). The Kaplan Meier curves reflect, in part, the confidence intervals that include zero, statistical significance that depends on a small number of patients, and the vision loss associated with treatment in some patients. Each of these limitations is further described below.
To ascertain whether the observed benefit of 117 days of delayed vision loss is the true effect one can look at the confidence intervals. The data, however, do not provide compelling evidence; given the broad range in the confidence intervals, the reported benefit may actually be one of 4 weeks or less rather than 3-4 months, or it may be zero- we cannot tell from the study.
It is important to emphasize that OPT with verteporfin is not a cure. Also, patients treated with verteporfin have already experienced vision loss and virtually all will continue to experience vision loss after treatment. Patients who receive verteporfin already have diminished vision and generally go on to experience continued loss of vision in spite of treatment. Any days of preserved vision that may result from treatment are not days of normal vision, but are days of moderately to severely limited visual function.
Confidence in the reported benefit must also take into account the actual number of patients who responded. In the VIP study (table 3, page 549), the actual numeric differences between treated and placebo groups were very small for all comparisons except for severe vision loss (i.e., ≥ 6-line decrease). Even then there was only a difference in 16 patients (VIP study, figure 3, page 548) between the verteporfin group who had severe vision loss (n=29) and the placebo group with severe vision loss (n=47). It is concerning that the statistical significance hinged on a small number of patients, and a different outcome in a few patients could potentially have changed the significance of the results.
Another important issue reflected in the Kaplan Meier curves is the risk of the treatment. The VIP article reported that 4.8% (8/166) of the occult with no classic patients treated with verteporfin suffered severe vision loss within seven days of the treatment, whereas none of the 92 placebo treated patients experienced such vision loss. Thus, this treatment has a non-trivial risk for a serious complication. This is of particular concern in this case because it is unclear if there is any clinical benefit from treatment, and if there is a benefit, it may be so small that it may not be sufficient to warrant exposing patients to this risk of accelerated severe vision loss.
The Kaplan Meier curves raise another question: why was the treatment effect not manifest until after the 12-month point? This was different from the findings in the TAP studies where an effect was seen by the one-year follow-up, and led us to question why the various types of CNV would respond differently. To help understand this more thoroughly, we asked the requestor to explain what the biological basis was for classifying CNV in AMD into different subtypes (i.e., predominantly classic, minimally classic, and occult with no classic).
The requestor stated that the differentiation of CNV fluorescein angiogram findings into classic and occult was in general ophthalmologic use prior to the TAP study as a result of the Macular Photocoagulation Study (MPS). The MPS was a multi-center, randomized clinical trial that investigated the use of photocoagulation in treating CNV in AMD patients.8 Experience from the MPS suggested that prognosis in AMD-associated CNV varied with the fluorescein angiogram appearance, and classic lesions were suspected of having a faster progression of vision loss as compared to occult CNV. Therefore, the investigators decided to study the effect of OPT with verteporfin in classic and occult CNV separately.
In the TAP study, which looked primarily at patients with classic CNV, 57 patients who had occult with no classic CNV were inadvertently included. Although the investigators reported a benefit from verteporfin treatment in the occult with no classic patients, the pattern of benefit seen was different from the pattern reported in the VIP study. In TAP, the 57 occult with no classic patients showed a statistically significant benefit in terms of moderate vision loss at 12 months as compared to placebo (p=0.02), whereas at 24 months the statistical support for a benefit was not significant (p=0.06). Conversely, in VIP, moderate vision loss difference was not significant between the verteporfin and placebo groups at 12 months (p=0.51), but was statistically significant at 24 months (p=0.032). Indeed, in the VIP study the greatest benefit was seen in regards to severe vision loss. The TAP study did not report the data for severe vision loss in their small subset of occult-only patients. Because it is unclear whether classic and occult CNV are pathophysiologically different, and the findings from the two studies showed that the occult-only patient group in the TAP study was small and behaved differently from the occult-only group in the VIP study, results of the TAP study cannot be used to bolster the results of the VIP study, and the VIP results must stand on their own.
Our October 17, 2001 Decision Memorandum considered only the data actually provided in the published report of the VIP study; we did not have the Kaplan Meier curves presented herein, nor did we have the confidence intervals listed in Table 3. In that memorandum, we did note that many verteporfin-treated patients still had lost vision at 24 months, but we also stated that a slight reduction in vision could result in clinical relevancy in a group, such as AMD patients with subfoveal CNV, in whom there is no good treatment alternative. However, the new data we have reviewed make it clear that while treatment with verteporfin may result in a temporary delay in vision loss, there are sufficient methodological issues to raise substantial uncertainty as to whether this small reported benefit is in fact real. The real benefit, if any, may only be a few weeks delay in vision loss. The unexpected time pattern for vision loss and the lack of clarity about the underlying pathophysiology of "wet" AMD subtypes, makes it more likely that the true effect, if any, lies at the lower end of the reported confidence intervals, possibly four or fewer weeks. This potential for a small benefit must be balanced against the real risk of sudden, severe vision loss.
Although there is no established treatment for subfoveal occult with no classic CNV in AMD, CMS believes that the available evidence is insufficient to demonstrate that OPT with verteporfin is clinically effective for these patients. The treatment must therefore still be considered experimental, and not reasonable and necessary. This conclusion is based on the failure to achieve significant benefit on the primary study outcomes, the lack of a clear biological rationale for different clinical effects for different variants of AMD, uncertainty about whether any benefit truly exists, and the severe complications associated with the experimental therapy.
CMS encourages the investigators to provide confirmatory data from a second adequately powered randomized clinical trial focusing solely on AMD patients who have occult with no classic subfoveal CNV. This decision is not meant to imply that we do not believe OPT with verteporfin could potentially be proven to be clinically effective in these patients; only that the evidence currently available does not support this conclusion.
IV. Summary of CMS Analysis
In summary, the scientific evidence is not adequate to conclude that OPT with verteporfin is clinically effective. The therapy must therefore still be considered experimental in the treatment of subfoveal occult with no classic CNV associated with AMD and thus not reasonable and necessary. Results from the VIP study show that OPT with verteporfin may slow the rate of progression of vision loss in occult with no classic CNV and may result in a potential, temporary delay of severe vision loss. However, methodologic and statistical issues (e.g., subgroup analyses, multiple comparisons, and confidence intervals that cross or approach zero) weaken our confidence that the observed effect of 117 days delayed vision loss is real. The VIP study shows clearly that there is no delay in deterioration of vision for the first year of treatment, and that virtually all patients continue to experience deteriorating vision whether treated or not. There are many unresolved questions such as the biologic rationale for treatment effect being dependent on the type of fluorescein angiogram pattern and why no effect with treatment was observed at 12 months. CMS encourages further study of the clinical effectiveness of this treatment in AMD patients who have subfoveal occult with no classic CNV. Until further studies confirm the VIP trial results, the use of OPT with verteporfin in this class of patients will remain a non-covered service.
V. Decision
The Centers for Medicare and Medicaid Services has decided to retain its national noncoverage policy of ocular photodynamic therapy with verteporfin in the treatment of subfoveal occult with no classic choroidal neovascularization associated with age-related macular degeneration.
This decision memorandum does not affect our national coverage policy for OPT with verteporfin for predominantly classic subfoveal CNV related to AMD. Other uses of OPT with verteporfin to treat AMD not already addressed by CMS will continue to be non-covered. These include the following AMD indications:
- Patients with minimally classic CNV lesions (where the area of classic CNV occupies < 50% of the area of the entire lesion);
- Patients with juxtafoveal or extrafoveal CNV lesions (lesions outside the fovea);
- Patients who are unable to obtain a fluorescein angiogram; and
- Patients with atrophic or "dry" AMD.
OPT with verteporfin for other ocular indications, such as pathologic myopia or the presumed ocular histoplasmosis syndrome, is not addressed in this decision memorandum nor in our national coverage policy, and continues to be eligible for coverage through individual contractor discretion.
1 Ryan S, et al. 1989.
2 Verteporfin in Photodynamic Therapy Study Group 2001.
3 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group 1999.
4 Treatment of Age-Related Macular Degeneration with Photodynamic Therapy Study Group 2001.
5 For detailed information on the study design of the TAP trial, please see http://www.cms.hhs.gov/coverage/8b3.asp
6 Verteporfin in Photodynamic Therapy Study Group, 2001.
7 Verteporfin in Photodynamic Therapy Study Group 2001.
8 Macular Photocoagulation Study Group 1991.


