To: Administrative File: CAG #00092N
C-Peptide Levels as a Criterion for Use of the Insulin Pump
From: Sean Tunis, MD, M.Sc.
Director of Coverage and Analysis Group
John Whyte, MD, MPH
Acting Director, Division of Items and Devices
Rebecca Ashkenazy
Policy Analyst, Division of Items and Devices
Betty White-Shaw;
Health Insurance Specialist, Division of Items and Devices
Sharon Hippler
Health Insurance Specialist, Division of Items and Devices
Mitchell Burken, MD
Acting Director, Division of Medical and Surgical Services
Subject: National Coverage Determination (NCD)
Date: May 11, 2001
This memorandum serves four purposes: (1) outlines the NCD on the continuous subcutaneous insulin infusion pumps for Type I diabetics [CAG-00041]; (2) discusses the use of C-peptide as a means to distinguish Type I vs Type II diabetics; (3) reviews the scientific and clinical literature on the use of C-peptide levels as a method of determining beta-cell activity; and (4) delineates the reasons for revising the C-peptide requirement for coverage of the pump.
Background
On September 24, 1999 the Health Care Financing Administration (HCFA) announced coverage for the use of continuous subcutaneous insulin infusion (CSII) pumps for Medicare recipients with Type I diabetes. 1 The decision requires that physicians document Type I diabetes with a C-peptide level of less than 0.5 ng/mL when requesting coverage for a continuous subcutaneous insulin infusion pump. (See Appendix A for complete criteria.) This C-peptide requirement was determined to be a reasonable method of distinguishing Type I versus Type II diabetes after a review of the scientific literature and discussion with clinicians, researchers, and various specialty societies. Within the past 12 months, we have heard from numerous physicians and patients who have requested that we reassess the C-peptide criterion for insulin pump coverage, since some clinically diagnosed Type I diabetics had C-peptide levels greater than 0.5 ng/mL, and otherwise would have qualified for coverage of the pump. In addition, we have also been asked to assess whether the C-peptide level of less that 0.5 ng/mL is an appropriate level to assess for Type I diabetics with End Stage Renal Disease (ESRD) and/or other renal dysfunction.

C-peptide is a polypeptide of 31 amino acids that acts as a structural connection within the proinsulin molecule. (Illustration reprinted with permission from Diagnostic Products Corporation www.dpcweb.com) It is released as a by-product when proinsulin, the precursor to insulin, is enzymatically cleaved to release insulin into the circulation. Insulin reserve, or the persistence of the ability to secrete insulin, is not an expected finding in Type I diabetes and low to undetectable levels of c-peptide should characterize the entity. Type II diabetics who require insulin should have a higher C-peptide level than Type I since Type II diabetes is frequently associated with insulin resistance as well as some insulin deficiency.
Although in theory, C-peptide level appears to be an appropriate means of differentiating the two types of diabetes, the discrimination between Type I and Type II diabetes has, in the past, been made solely on clinical grounds. According to the Expert Committee on the Diagnosis and Classification of Diabetes (an international expert committee established in 1995 under the sponsorship of the American Diabetes Association), Type I diabetes is characterized by beta-cell destruction usually leading to absolute insulin deficiency. 2 The rate of beta-cell destruction is variable and at later stages of the disease there is little or no insulin secretion, "as manifested by low or undetectable levels of plasma C-peptide." 3
Distinguishing between Type I and Type II diabetes is a recognized area of medical controversy. There is no established laboratory or clinical algorithm to make a definitive diagnosis. While there are clinical characteristics that can suggest either entity, there are no formal criteria. For example, ketoacidosis is associated with Type I diabetes in children and adolescents, but has only a 14% incidence in teens with Type II diabetics. 4 Other characteristics that distinguish between Type I and Type II are the presence or absence of obesity, age of onset, acanthosis nigricans and a first-degree relative with diabetes.
Reference values for normal C-peptide levels
Unlike many other laboratory tests, there can be several reference ranges for C-peptide levels, especially depending upon type of laboratory assay used, age of patient, and whether or not a patient has fasted prior to the test. There are two laboratories processes routinely used to quantify C-peptide.5 These include:
- radioimmunoassay (RIA) method and
- immunochemiluminometric assay (ICMA) method.
In the RIA method, C-peptide is measured using goat anti-C-peptide. The antibody also recognizes proinsulin but has no crossreactivity with insulin. The analytic sensitivity of the test is 0.125 ng/ml and an overnight fast is required. The normal reference range for normal adults is 0.5 - 2 ng/mL. 6
In the ICMA method, a competitive immunoassay with two, 30-minute incubation cycles are used. The analytic sensitivity of the test is 0.3 ng/mL. The normal reference value for this test is 0.9 - 4 ng/mL and the patient must be fasting. This reference was obtained from the results of serum samples from 35 fasting laboratory volunteers. Specific age-related reference ranges are presented in Table 1. 7
Table 1: Specific age-related reference ranges
for plasma-c-peptide (ICMA method)
| Age |
C-peptide value (ng/mL) |
| birth - 9 years and 11 months |
0.0-0.3 |
| 10 years - 16 years and 11 months |
0.4-3.3 |
| 17 years and up |
0.9 - 4 |
C-peptide levels may also vary depending upon stage of the disease. According to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, those patients with immune-mediated diabetes "eventually become dependent on insulin for survival and are at risk for ketoacidosis. At this latter stage of the disease, there is little or no insulin, as manifested by low or undetectable levels of plasma C-peptide."
Summary of Evidence
Scientific Studies
The medical literature was searched using OVID and PubMed. Search terms included "C-peptide", "Type 1 Diabetes", "Type 2 Diabetes", "NIDDM" and "IDDM." A total of nine studies were reviewed. Note that studies vary in terms of units used to express C-peptide level. In general, ng/mL x 0.331 = nmol/L.
Welborn et al. (1981) designed a study to determine diabetes type by measuring insulin secretory capacity. Looking at 170 hospital clinic diabetics and 140 country survey diabetics, they obtained c-peptide measurements, fasting and postprandial. 8 (Measurements were taken with a double antibody immunoassay to synthetic human C-peptide M1230 with a limit analytic sensitivity of .02 nmol/L [.06 ng/ml]; fasting level for serum C-peptide by this method in adults is 0.55 nmol/L, range 0.20-1.0 nmol/L) Subjects with known renal failure were excluded and final sample from 107 hospital clinic diabetics and 94 country survey diabetics were analyzed. Utilizing a c-peptide cutoff of .16 nmol/L (.48 ng/mL) they stratified patients into two groups: one characterized by exclusive insulin requirement, lean body mass, and young age of onset and the other by a high proportion on diet or oral therapy, tendency to obesity, and age of onset beyond 40 years. There were 11 subjects with a c-peptide ranging from .17 to .32 nmol/L (.51 to .966 ng/mL) that represented an "indeterminate status." They suggest utilizing a C-peptide level of .16 nmol/L (.48 ng/mL) to segregate those with characteristics of Type I diabetes and Type II diabetes. They further noted that the cut-off point selected provides high specificity for insulin requirement.
Katzeff et al. (1985) studied 20 diabetic patients to determine whether individual subjects with Type I diabetes or Type II diabetes, all treated with insulin, could be reliably distinguished with basal C-peptide. 9 The study compared 10 Pima Indians with early onset diabetes to 10 Caucasoids with early onset diabetes. All patients had developed diabetes before 21 years of age and were receiving insulin treatment. Previous studies had demonstrated that the early diabetic onset in the Pima population was most likely Type II and in the Caucasoid population most likely Type 1. Utilizing a radioimmunoassay method, the study detected the mean C-peptide level in the Caucasoid group of .02 .01 nmol/L (.06 .03 ng/mL) versus .73 .17 nmol/L (2.2 .51 ng/mL) in the Pima Indians [p<0.001]. However, there was some overlap between the two groups in terms of their c-peptide levels. The authors concluded that among patients with early onset diabetes mellitus who have been treated with insulin, C-peptide measurements in fasting blood or in 24-hour urine collections can be used to categorize subjects without renal failure into insulin-dependent and non-insulin-dependent groups even after many years of diabetes.
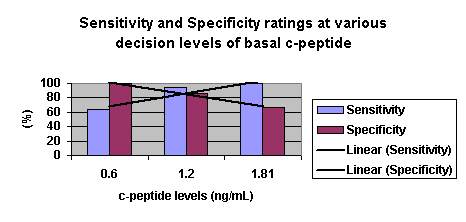
Koskinen et al. (1985) studied the basal and stimulated C-peptide concentrations among newly and previously diagnosed diabetics (n=105) hospitalized in Tuku University Hospital for one year. 10Their objective was to investigate the usefulness of C-peptide determination as a criterion predicting adequate treatment of diabetes mellitus for adults. Of those requiring insulin, mean c-peptide levels were .13 .14 nmol/L. [.393 .422 ng/mL]. Of non-insulin requiring diabetics mean levels were 1.04 .74 nmol/L [3.14 ng/mL]. The study concluded that a high sensitivity for the detection of the need of insulin therapy would be obtained using a basal c-peptide level of .6 nmol/L [1.81 ng/mL]. They note, however, that this criterion lacks acceptable specificity. They also concluded that a post-stimulatory C-peptide concentration of .6 nmol/L [1.81 ng/mL] proved to be a reliable for choice of therapy. The data indicating the sensitivity and specificity ratings at various decision levels for basal C-peptide is presented in Table 2.
Table 2: Sensitivity and Specificity at various decision levels for basal C-peptide (Koskinen, 1985)
|
|
Basal C-peptide cut-off |
Basal C-peptide cut-off |
Basal C-peptide cut-off |
|
|
.2 (nmol/L)
[.6 ng/mL] |
.4 (nmol/L)
[1.21 ng/mL] |
.6 (nmol/L)
[1.81 ng/mL] |
| Sensitivity (%) |
Previously diagnosed DM |
68 |
92 |
100 |
|
Newly Diagnosed DM |
56 |
100 |
100 |
|
All included cases |
64 |
95 |
100 |
| Specificity (%) |
Previously diagnosed DM |
100 |
86 |
73 |
|
Newly Diagnosed DM |
100 |
83 |
50 |
|
All |
100 |
86 |
67 |
There is an inverse relationship between sensitivity and specificity, and given increased C-peptide cut-off levels the specificity of the test decreases while the sensitivity increases.11
Kyllastinen and Lefving (1986) looked at 121 elderly subjects, of which 25 were non-diabetic controls ages 69-86 years, and 96 were type 2 (NIDDM) diabetics ages 64-96 years. 12 They evaluated basal c-peptide levels in the aged and clarified their practical value in assessing whether elderly diabetic patients benefit from insulin therapy or not. Fasting serum c-peptide concentrations were .51 .2 nmol/L [1.54 .6 ng/mL] for controls; .6 .16 nmol/L [1.81 .48 ng/mL] for diabetics on diet alone; .72 .33 nmol/L [2.17 1 ng/mL] for diabetics on tablets and .44 .23 nmol/L [1.33 .69 ng/mL] for diabetics on insulin (p < .001 for diabetics on tables versus controls and diabetics on tablets vs. diabetics on insulin). The result demonstrated a wide range of basal c-peptide concentration in elderly diabetics on different treatment. They concluded that "very low levels were found only in diabetics treated with insulin; and it is obvious that patients with a disease resembling type 1 diabetics are included in this group."
Damsgaard (1987) in a study of a Danish population of 5699 individuals (60-74 years old), looked at the use of c-peptide to classify patients with insulin-treated diabetes. 13 Of this population screened by fasting blood glucose and a diabetes interview, 236 had a history of diabetes. Fasting c-peptide in all known subjects without insulin treatment was > .29 pmol/mL [.876 ng/mL]; and 95% had greater than .38 pmol/mL [1.14 ng/mL]. Those known diabetics treated with insulin had wide ranges of fasting c-peptide. Fasting C-peptide greater than .30 pmol/mL [.906 ng/mL] was found in 32 (61.5% of insulin-treated known diabetics; 95% of the values were greater than >.06 pmol/mL [.18 ng/mL]). Of the 52 known diabetics treated with insulin, only 20 had C-peptide values in the insulin-dependent range. The authors concluded that lower limits for non-insulin dependent diabetes of .30 pmol/mL [.906 ng/mL] for fasting c-peptide "seems reasonable." An overlap between NIDDM and IDDM was observed for fasting c-peptide between .20 and .40 pmol/mL. [.60 and 1.21 ng/mL, respectively]
Gjessing et al. (1989) examined 132 insulin treated subjects greater than age 18 to study fasting plasma C-peptide, glucagon stimulated plasma c-peptide, and 24 h urinary C-peptide in relation to clinical type of diabetes.14 (All those with elevated plasma creatinine were excluded.) Utilizing a radioimmunoassay method, the percentage predictive value of a negative fasting C-peptide test, defined as less than .2 nmol/L [.6 ng/mL], was 86%. The authors noted an overlap in C-peptide values between patients with Type 1 and Type 2 diabetes and that ten percent of those with a plasma C-peptide <.06 nmol/L [.18 ng/mL] were classified as Type 2 diabetics clinically. The authors concluded that plasma c-peptide after glucagon stimulation and basal C-peptide give a good discrimination between clinical Type 1 and Type 2 diabetes, while 24 h urinary c-peptide seems to be less sensitive in this discrimination. Gjessing also indicated that plasma c-peptide values depend on clearance, technical procedures, and during unsteady state conditions also on c peptide volume of distribution. He reported that generally values < 0.2 nmol/l [0.6 ng/mL] suggest an insulin requirement, while values >0.5nmol/l suggest non-insulin requirement.
Landin-Olsson et al. (1990) looked at 244 consecutive patients diagnosed with diabetes mellitus during two years at Malmo, Sweden. 15They obtained data including age, body mass index, HbA1c, c-peptide, and level of islet cell antibodies at clinical onset and after a follow-up (n=233) after a median time of 31 (range 1 - 49) months. C-peptide was measured by radioimmunoassay with a lower detection limit of 0.10 nmol/L with reference values 0.25-0.75nmol/l. They found in patients initially treated with insulin (n=42) fasting c-peptide levels ranged from .1 to 1.54 nmol/L [.3 ng/mL to 4.97 ng/mL]. In those not initially treated with insulin fasting c-peptide ranged from .1 to 4.1 nmol/l [.30 to 12.3 ng/mL]. The study concluded that the sensitivity, specificity, and predictive value for insulin treatment for low c-peptide value <.25 nmol/L [.755 ng/mL] was 60%, 96%, and 80% respectively.
Scott et al.(1996) attempted to describe the characteristics of youth-onset NIDDM at diagnosis as compared to youths with IDDM. 16 Looking specifically at 40 non-insulin dependent (NIDDM) diabetes with 48 insulin-dependent diabetes (IDDM), the authors illustrated that the mean c-peptide (ng/mL) levels was 4 1.0 and 0.8 .2, respectively. The reference range used for normal was .4 to 2.4 ng/mL.
Torn et al. (2000) looked at a 2-year follow-up of 281 patients (ages 15-34) who were diagnosed with diabetes between 1992 and 1993. 17 The authors noted that at diagnosis, C-peptide levels were lower (.27; .16-.4 nmol/L) [.81; .48 - 1.21 ng/mL] in autoantibody-positive patients compared with autoantibody-negative patients (.51; .28-.78 nmol/L) [1.54; .84-2.35 ng/mL] (p < .001) After 2 years, C-peptide levels had decreased significantly in patients with autoimmune diabetes (.20; .1 - .37 nmol/L; p = .0018) [.6; .3-1.11 ng/mL], but not in autoantibody-negative patients.
Wright et al (2000) studied 21 insulin-using patients who had been labeled by their physicians as having IDDM. 18 Using the clinical criteria of the National Diabetes Data Group, only thirteen were found to have IDDM. Using fasting C-peptide values ( <0.26 nmol/L to indicate insulin deficiency), only 5 of the 13 truly had IDDM. The authors concluded that "the clinical classification of diabetes mellitus may be inaccurate and that C-peptide evaluation improves the accuracy of the classification."
Utility of C-peptide in Patients with Renal Dysfunction
The kidney is the major site for C-peptide catabolism and excretion. In theory, those patients with renal dysfunction should have elevated C-peptide levels, since decreased urinary excretion would lead to elevated plasma levels. It is unclear, however, how much higher these levels should be. Researchers have come to different conclusions regarding the utility of assessing c-peptide levels in patients with renal dysfunction.
Katseff et al. (1985) studied 20 Type 1 and Type 2 diabetic patients and concluded if renal failure is present, fasting C-peptide level may be elevated. 19If this is suspected the renal clearance of c-peptide can be determined, and if decreased, both the serum and urinary concentration should be considered unreliable as indices of insulin secretion.
Benhamou et al. (1992), in a retrospective study of 472 patients on chronic hemodialysis, concluded that fasting plasma c-peptide is of good value for the classification of patients with end-stage renal disease and can help in the management of therapy. They compared the classification by a diabetologist with a cut-off for C-peptide of less than or equal to .6 ng/ml as a negative c-peptide (using radioimmunoassay method). In this method, three patients were discordant for classification between the diabetologist and c-peptide level. The predictive value of a "negative c-peptide" and "positive c-peptide" were 100% and 96% respectively.20 Of note, none of these patients were using a continuous subcutaneous insulin infusion pump.
Covic et al. (2000), enrolling 341 diabetic ESRD patients from northeastern Ohio to assess if their C-peptide concentrations accurately reflected their insulin synthesis. 21 The goal was to specifically identify patients with type 2 diabetes. They compared patient clinical diagnosis (as stated on the Health Care Financing Administration (HCFA) 2728 forms) to a diagnosis utilizing basal and stimulate c-peptide values. Looking at 127 patients (additional criteria included a family history of diabetes and at least one living diabetic sibling having been phenotyped and genotyped), they discovered that using clinical criteria 79% of the study population were categorized as type 1 (10%) or type 2 diabetics (69%), while 21% of diabetic ESRD patients could not be classified. Ninety-eight percent of the patients were classified as type 2 diabetics when stratified by c-peptide concentrations using criteria derived the Diabetes Control and Complications Trial Research Group (DCCT). With clinical phenotyping criteria as the standard for comparison, C-peptide concentrations classified diabetic ESRD patients with 100% sensitivity, but only 5% specificity. Covic concluded that although 10% of the diabetic ESRD study population were classified as type 1 diabetics using clinical criteria, only 1.5% of these patients had c-peptide levels less than .2 nmol/L [.6 ng/mL] (the standard they used to discriminate type 1 from type 2 diabetes in patient with normal renal function.) They found that approximately 85% of diabetic ESRD patients in the study who were classified as type I diabetics using clinical criteria had c-peptide values indistinguishable from type 2 diabetics. In the study the criteria of c-peptide concentrations greater than .5 nmol/L [1.5 ng/ml] and diabetes onset in patients who are more than 38 years old identify type 2 diabetes with a 97% predictive value. They directly contrasted their results to those of Benhamous stating that their result may be discrepant because the racial composition of the study and varying clinical classification schemes.
Position Statements
Neither the American College of Endocrinology (ACE), nor the American Diabetes Association (ADA) has an official position statement on the use of C-peptide as a criterion for distinguishing between Type I and Type II diabetics, or as a requirement for the use of an insulin pump. However, both societies have written to the agency on this topic.
In a March 26, 2001 letter to HCFA, Helena Rodbard, MD, FACE, Chair of the American Association of Clinical Endocrinologist's Task Force on Insulin Pump Coverage suggested the following revisions to the present policy:
- Broaden the limits of eligibility for coverage of insulin pumps and supplies to include patients with C-peptide levels up to 1.0 ng/mL
- Eliminate the distinction between type 1 and type 2 diabetes in the eligibility requirements
- Allow for higher levels of C peptide for patients with chronic renal insufficiency up to 2.0, with a serum creatinine > 1.8 mg/dL
- Exemptions to the eligibility requirements should include those patients with a documented hospital visit for DKA or hypoglycemia. Patients who have been on an insulin pump for at least six months at the time of Medicare eligibility should also be exempt from the coverage requirement.
In a March 21, 2001 letter to HCFA, Marian Parrott, MD, MPH, Vice President, Clinical Affairs of the ADA, stated "the role of C-peptide assay is unclear.....It is difficult to suggest a cut point that will include all patients with Type I diabetes while excluding all patients with type II. However, if an objective cut point were essential, then a level between 0.8 ng/ml and 1.0 ng/ml would probably serve to include virtually all type I patients. The number of individuals with type 2 would then be eligible for pump therapy should be small, as patients would still need to meet clinical criteria..." In addition, the ADA reported in a January 29, 2001 note that most studies relating to pump use require a C-peptide < 0.8 ng/mL for enrollment.
HCFA Analysis
In determining the usefulness and appropriateness of C-peptide as a criterion for the continuous subcutaneous insulin infusion pump, HCFA addressed the following analytic questions:
- What are the reference values for normal C-peptide levels? How sensitive are the various measurement tests?
- What is the appropriate value for making the distinction between Type I and Type II based on C-peptide?
- Are there certain circumstances (e.g. renal insufficiency, early-onset diabetes) that will cause a higher-than-expected C-peptide level?
As noted earlier, there is significant variability in "normal" values for C-peptide, that depend on measurement method used, age, onset of disease, renal insufficiency, and fasting status. In the RIA method, assuming all other variables adjusted, 0.5 ng/mL might have been appropriate as an upper threshold for coverage, since this value was the lower end of normal. For the ICMA method, 0.5 ng/mL would not have been high enough, since the lower end of normal for this test is 0.9 ng/mL. Since the C-peptide level is an absolute requirement for Medicare coverage of insulin pumps, there likely needs to be a recognition that these tests do have a certain degree of error. The sensitivity of the tests is fairly good, and is as accurate as most other laboratory tests. However, given standard error of any test, 0.5 ng/mL is probably too low, and may exclude some patients who truly are insulin deficient. Given that the sensitivity and specificity of the test is approximately 90%, a 10% variability is reasonable given the precision of laboratory tests.
Clearly, C-peptide level does provide useful information. Although not definitive, numerous studies demonstrated that C-peptide level distinguishes Type I from Type II, as well as indicates the need for insulin. Table 4 provides a summary of various C-peptide "cut-off values" from the various studies reviewed. Values range from 0.6 ng/mL to 1.8 ng/mL, again varying based on measurement method.
Table 4: Summary of lower proposed C-peptide cut-off values for discrimination between diabetes types
|
Function of C-peptide cut-off |
C-peptide cut-off (method) |
| Welborn et al. (1981) |
Separate clinical characteristics of diabetes type |
.16 nmol/L [.48 ng/mL]
(RIA) |
| Koskinen et al. (1985) |
Discriminate insulin requirement |
.6 nmol/L [1.81 ng/mL]
(RIA) |
| Damsgaard et al. (1987) |
Classification of patients with insulin treated diabetes |
.30 pmol/ml [.906 ng/mL] |
| Gjessing et al. (1989) |
Predictive value of c-peptide as indicator of Type 2 and Type 1 diabetes |
.2 nmol/L [.6 ng/mL]
(RIA) |
| Landin-Ollson (1990) |
Marker to predict insulin dependence |
.25 nmol/L [.755 ng/mL]
(RIA) |
As with any diagnostic test, one must balance sensitivity and specificity. As Figure 1 demonstrates, there is an inverse relationship between sensitivity and specificity; given higher C-peptide levels, the specificity decreases while the sensitivity increases. 22 As a result, we will include more patients which we believe are Type I diabetics, although there may be more false positives. Given the morbidity of diabetes, it may be preferable to allow more false positives than false negatives, especially since patients will have to meet several other criteria before coverage is allowed.
It is important to note that when HCFA instituted a C-peptide requirement < 0.5 ng/mL, we were not fully cognizant that the normal reference ranges vary significantly by measurement method. We chose 0.5 ng/mL since that was the lower limit of normal in the RIA method. A level of 0.6-0.9 ng/mL is still below normal in the ICMA method, but patients would be denied coverage of the pump, since the C-peptide level was above what we incorrectly perceived to be the lower limit of normal for all measurement methods. A review of several claims demonstrate that the majority of claims denied for C-peptide levels > 0.5 ng/mL were performed using the ICMA method. The current trend is for greater use of the ICMA method, since it appears to be technically more practical, and reagents have a longer shelf-life. Most of the scientific literature uses RIA since it is a more sensitive test. These different lab test ranges do not compare directly to each other.
Although there is debate about the precise value, C-peptide levels still remain very useful in providing information on beta-cell activity. There was greater consistency in the scientific literature over the role of C-peptide in determining whether or not a patient needs to begin an insulin regimen. If a C-peptide level is below the lower end of normal from a particular lab, there is little beta-cell activity. Given that data, it is worthwhile to rethink the issue of using C-peptide levels to distinguish Type I from Type II diabetics. For those insulin-requiring Type II diabetics that have a C-peptide less than the lower limit of normal, their physiology is now more of insulin -deficiency, the "burned-out pancreas" population. Such patients are now acting similar to a Type I diabetic. This is a very small number of patients, most likely less than 5 % of insulin requiring Type II diabetics. This population could benefit from an intensive insulin regimen, which includes a continuous subcutaneous insulin infusion pump. Presently, such patients would not be eligible for a insulin pump, since Medicare's coverage requires the patient to be a Type I diabetic. Since C-peptide gives reliable data on beta-cell activity, and therefore insulin requirements, we will remove the Type I requirement for an insulin pump, and allow insulin-requiring Type II diabetics to receive the pump, if they meet all other coverage criteria as well as have a documented C-peptide level less the lower limit of normal. Given some imprecision of the tests, as well as the fact that the tests are not 100% accurate in terms of specificity and sensitivity, 10% variation in the test will be allowed.
There is debate as to why not remove the C-peptide requirement completely, and allow the physician to make the distinction between Type I and Type II. It would be premature to remove the C-peptide requirement for several reasons: (1) For those Type I diabetics with higher C peptide levels above a lab's reference range, they most likely have residual beta-cell function. The extent to which aggressive insulin therapy alters the natural history of residual insulin secretion in Type I diabetes has not been firmly established, and it would be premature to allow an intensive insulin regimen without further study; (2) Since the physiology of the diabetes varies between Type I and Type II, we did not believe that one could extrapolate conclusions from data on patients with Type I diabetes to patients with Type II diabetes. It is still unclear as to the precise role of an intensive insulin regimen for all insulin-requiring Type II diabetics. C-peptide levels provide the necessary reliability and validity. Although imprecise, they are still useful. We are willing to revisit this issue as more information becomes available, either on the role of an intensive insulin regimen for Type II diabetes, or more information on the variability of C-peptide measurements.
As noted earlier, fasting status, onset of disease, and renal insufficiency all seem to affect, in some way, C-peptide levels. The reference ranges for labs quoted in the text of this document are for fasting individuals; therefore, we will specify that C-peptide level should be performed in a fasting patients. As for onset of disease, if C-peptide level is elevated, it is most likely due to residual beta-cell activity, and it is unclear as to the appropriateness of an intensive insulin regimen through the use of pump, at that point in time. As for patients with ESRD, it remains unclear as to the appropriateness of an insulin infusion pump for ESRD patients undergoing dialysis. When the kidney has progressed to failure, insulin requirements often decrease, and there may be less need for an intensive insulin regimen. For those patients with chronic renal insufficiency, although in theory, the C-peptide level should be elevated, there is little data to support this belief, and the studies are contradictory.
Conclusion
The use of C-peptide appears to be a rational means of discriminating residual beta-cell function. There is consensus that Type I diabetes leads to beta-cell destruction and absolute insulin deficiency, with a resultant low C-peptide level. Such a patient population could benefit from an intensive insulin regimen, including a subcutaneous insulin infusion pump, and the evidence currently supports such pumps for Type I diabetes. In order to facilitate the diagnosis of Type I diabetes, we require a C-peptide level with the understanding that although it is not a single determinant of diabetes status, it does provide a measure of insulin secretory ability and consequent appropriateness of the infusion pump. The difficulty lies in the specific C-peptide value that distinguishes between Type I and Type II diabetes; a specific discriminating C-peptide level has not been rigorously tested, although in general, most laboratory tests do a very good job of accurately measuring the level of C-peptide. As a result, we will remove the 0.5 ng/mL requirement, and substitute a fasting C-peptide requirement that is less than, or equal to, the lower limit of normal of whichever laboratory test a physician chooses, 10% in the value to account for the small imprecision of the test. For example, in the RIA method, this would roughly translate to 0.6 ng/mL and for ICMA method, it would be approximately 1.0 ng/mL. This revision and allowance for a small error will enhance the test's ability to include more Type I diabetics, as well as include some insulin-requiring Type II diabetics that have little residual beta-cell activity, and therefore are acting more like Type I diabetics.
At this point in time, it is still not clear on the precise role of insulin pumps for Type II diabetics, although some data is encouraging. The etiology of diseases between Type I and Type II diabetes is significantly different and the effects of intensive insulin treatment need to be determined. At the same time, however, there are a few insulin-requiring Type II diabetics, who have a "burned out" pancreas, and therefore could benefit from a subcutaneous insulin infusion pump, and thus will be covered if they meet all other requirements.
The impact of renal dysfunction on c-peptide values has yet to be rigorously tested, therefore, no further adjustments will be made in the C-peptide value. No exceptions will be made in the policy at this time for ESRD patients.
DECISION:
Revise Coverage Issues Manual 60-14, so that:
- Adjust Fasting C-peptide requirement such that the value must be less than or equal to, the lower limit of normal of the lab's measurement method, 10%.
- Remove the Type I requirement, to include Type II diabetics as long as they meet all other requirements, including fasting C-peptide requirement, as noted above.
COVERAGE ISSUES - DURABLE MEDICAL EQUIPMENT 60-14
60-14 INFUSION PUMPS
THE FOLLOWING INDICATIONS FOR TREATMENT USING INFUSION PUMPS ARE COVERED UNDER MEDICARE:
External Infusion Pumps.
Continuous subcutaneous insulin infusion pumps (CSII) (Effective for Services Performed On or After 4/1/2000).
An external infusion pump and related drugs/ supplies will be covered as medically necessary in the home setting in the following situation: Treatment of Type I diabetes
In order to be covered, patients must meet criterion A or B:
- The patient has completed a comprehensive diabetes education program, and has been on a program of multiple daily injections of insulin (i.e. at least 3 injections per day), with frequent self-adjustments of insulin dose for at least 6 months prior to initiation of the insulin pump, and has documented frequency of glucose self-testing an average of at least 4 times per day during the 2 months prior to initiation of the insulin pump, and meets one or more of the following criteria while on the multiple daily injection regimen:
- Glycosylated hemoglobin level(HbAlc) > 7.0%
- History of recurring hypoglycemia
- Wide fluctuations in blood glucose before mealtime
- Dawn phenomenon with fasting blood sugars frequently exceeding 200 mg/dl
- History of severe glycemic excursions
- The patient with Type I diabetes has been on a pump prior to enrollment in Medicare and has documented frequency of glucose self-testing an average of at least 4 times per day during the month prior to Medicare enrollment.
Type I diabetes needs to be documented by a C-peptide level < 0.5
Continued coverage of the insulin pump would require that the patient has been seen and evaluated by the treating physician at least every 3 months.
The pump must be ordered by and follow-up care of the patient must be managed by a physician who manages multiple patients with CSII and who works closely with a team including nurses, diabetes educators, and dietitians who are knowledgeable in the use of CSII.
Subcutaneous insulin infusion pumps will continue to be denied as not medically necessary and reasonable for all Type II diabetics including insulin-requiring Type II diabetics.
1 See CAG-00041 or visit www.cms.hhs.gov/coverage
2 Report on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-1195.
3 Report on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-1195.
4 Kaufman, FR. Diabetes in Children and Adolescents Areas of Controversy. Medical Clinics of North America 1998;82:721-738.
5 Both tests assume a normal distribution
6 Information provided by Quest Diagnostics
7 Information provided by LabCorp
8 Welborn TA, Garcia-Webb P, Bonser AM. Basal C-peptide in the discrimination of type I from type II diabetes. Diabetes Care 1981;4:616-619.
9 Katzeff HL, Savage PJ, Barclay-White B, et al. C-peptide measurement in the differentiation of type I (insulin-dependent) and type 2 (non-insulin dependent) diabetes mellitus. Diabetologica 1985;28:264-268.
10 Koskinen P, Viikari J, Irjala K. Kaihola H, Seppala. C-peptide determination in the choice of treatment in diabetes mellitus Scan J Clin Lab Invest 1985; 45: 589-597.
11 In this example, sensitivity would be defined as the proportion of diabetics who are Type I who have a positive test (low C-peptide level). A highly sensitive test will rarely miss people with Type I who have a low C-peptide level. Specificity is the proportion of diabetics who are Type II with a negative test, i.e. higher C-peptide level. A specific test will rarely misclassify people.
12 Kyllastinen M, Elfving S. Serum C-peptide concentrations and their value in evaluating the usefulness of insulin therapy in elderly diabetics. Gerontology 1986; 32: 317-326.
13 Damsgaard EM, Faber OK, Froland A, Green A, Hauge M, Hom, NV, Iversen S. Prevalence of Fasting Hyperglycemia and known non-insulin-dependent diabetes mellitus classified by plasma c-peptide: Frederica Survey of Subjects 60-74 Yr Old. Diabetes Care 1987;10: 26-32.
14 Gjessing HJ, Matzen LE, Faber OK, Froland A. Fasting plasma c-peptide, glucagon stimulated c-peptide and urinary c-peptide in relation to clinical type of diabetes. Diabetologia 1989; 32: 305-11.
15 Landin-Olsson. M, Nilsson KO, Lernmark A Sundkvist G. Islet cell antibodies and fasting c-peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologica 1990;33:561-568.
16 Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristic of Youth-onset Non-insulin-dependent Diabetes Mellitus and Insulin -dependent Diabetes Mellitus at Diagnosis. Pediatrics 1997;100:84-91.
17 Torn et al. Prognostic Factors for the Course of [Beta] Cell function in autoimmune diabetes. Journal of Clinical Endocrinology & Metabolism. 2000;85: 4619-4623.
18 Wright-Pascoe R, Mills J, et al. The role of C-peptide in the classification of diabetes mellitus. West Indian Medical Journal 2000;49:138-142.
19 Katzeff HL, Savage PJ, Barclay-White B, et al. C-peptide measurement in the differentiation of type I (insulin-dependent) and type 2 (non-insulin dependent) diabetes mellitus. Diabetologica 1985;28:264-268.
20 Benhamou et al. Classification of diabetes in patients with end-stage renal disease. Clin Nephrol 1992; 38: 239-44.
21 Covic et al. Serum C-peptide concentrations poorly phenotype type 2 diabetic end-stage renal disease patients. Kidney International 2000;58:17420-1750.
22 High sensitivity is typically used to rule out disease, while high specificity is used to rule in disease.


