TO: Administrative File: CAG-00431R
SUBJECT: Proposed National Coverage Determination Reconsideration forBeta Amyloid Positron Emission Tomography in Dementia and Neurodegenerative Disease
DATE: July 17, 2023
I. Proposed Decision
The Centers for Medicare & Medicaid Services (CMS) is proposing to remove the national coverage determination (NCD) at § 220.6.20, ending coverage with evidence development (CED) for positron emission tomography (PET) beta amyloid imaging and permitting Medicare coverage determinations for PET beta amyloid imaging to be made by the Medicare Administrative Contractors (MACs) under § 1862(a)(1)(A) of the Social Security Act (the Act).
II. Background
Throughout this document we use numerous acronyms, some of which are not defined as they are presented in direct quotations. Please find below a list of these acronyms and corresponding full terminology:
AD – Alzheimer’s disease
Aβ – Amyloid-beta, used interchangeably with beta amyloid
CED – Coverage with Evidence Development
CMS – Centers for Medicare & Medicaid Services
FDA – Food and Drug Administration
NCA – National Coverage Analysis
NCD – National Coverage Determination
PET – Positron Emission Tomography
US – United States
Alzheimer’s disease and the amyloid hypothesis
Alzheimer’s disease (AD) is a currently irreversible, fatal brain disorder that progressively degrades memory, cognitive function (thinking and reasoning), and eventually motor function. With >6 million individuals afflicted, AD is the number one cause of dementia in older Americans, and one of the most burdensome diseases for the Medicare population. Although older Blacks and Hispanics are more likely than Whites to have AD (Blacks twice as likely, Hispanics one and one-half times as likely), minorities have been markedly underrepresented in all Alzheimer’s-related research, until very recently. (AA 2022, NIA 2022, CDC 2022, Rajan 2021, Brookmeyer 2018, 2019.)
Most individuals with AD become symptomatic after age 65. The progression from mild cognitive impairment (MCI) to mild dementia is marked by involvement of more than one cognitive domain and substantial interference with daily life (Knopman 2014, Petersen 2018). Most patients with AD have other neurogenerative diseases as well that contribute to their progressive cognitive decline (Schneider 2007, Wilson 2010, Brookmeyer 2018), which further complicates diagnosis and potential treatment. For more detail on AD epidemiology, stages of disease, mixed disease, and potential treatments, see NCD 200.3, Monoclonal Antibodies Directed Against Amyloid for the Treatment of AD, which went into effect on April 7, 2022 (available at https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305).
The underlying cause(s) of AD remain unknown. Prominent risk factors include genetic predisposition (e.g., the apolipoprotein ε 4 allele, or APOE-ε4), family history of dementia, and risk factors for cardiovascular disease (e.g., high blood pressure, lack of exercise, diabetes, obesity, smoking) (CDC 2022, NIA 2022, AA 2022, CMS 2013).
Molecular biomarkers considered hallmarks of AD are amyloid-beta (Aβ) plaques, and neurofibrillary tangles of the protein tau. Neurodegeneration, evidenced by atrophy in specific brain regions on MRI, is a less specific marker, but correlates better with clinical progression of disease; as such, it is the downstream neuronal dysfunction and loss that appear to cause the symptoms of Alzheimer’s disease (Jack 2018, Jack 2011).
Investigators hypothesize that a wide range of factors may contribute to the development of AD, including genetic, metabolic, inflammatory and immune system, mitochondrial, environmental, and neuronal, to include both cytoskeletal (occurring within the neuronal cell itself, like tau) and synaptic (altering the connectivity among neurons, like Aβ plaques). (McAlpine 2021, ECRI 2012, Pimplikar 2009, Herrup 2010, Sperling 2011.)
While amyloid may have normal functions in the body and brain, abnormal amyloid is the first known physiological change found in AD patients, giving rise to the amyloid cascade hypothesis. The hypothesis posits a causal role for amyloid as instigator or essential component of a common pathway (the "central event in the aetiology of Alzheimer's disease") that leads to downstream changes including inflammation, tau pathology, and ultimately neurodegeneration. (Alzheimer 1898, 1907; Glenner 1984, Goate 1991, Hardy 1991, Selkoe 1991, Beyreuther 1991, Selkoe and Hardy 2016).
Recently, the amyloid hypothesis was under heightened public scrutiny because of reports questioning the integrity of some research publications on the underlying science (Pillar 2022), and related, ongoing investigations. However, the larger question surrounding the amyloid hypothesis – really since its inception – has not been whether Aβ is a hallmark of Alzheimer’s (it is), but whether, or to what degree, Aβ is a cause versus marker of the disease (CMS NCD 200.3 at https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305).
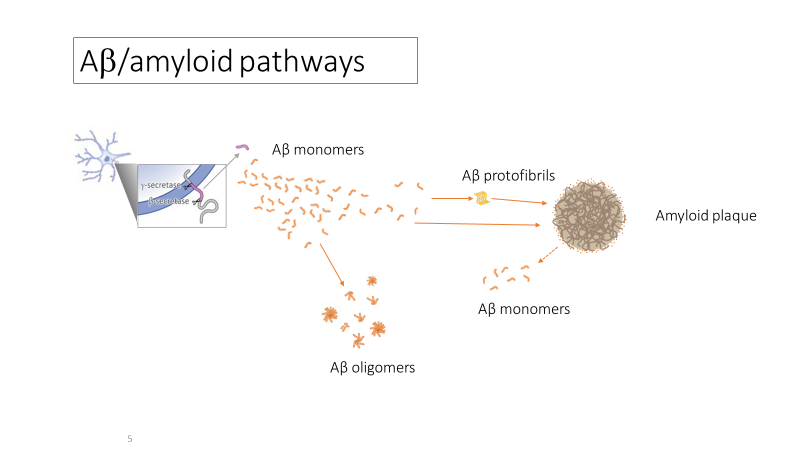
Further, scientists who find the amyloid hypothesis compelling nonetheless disagree about which form (or “species”) of Aβ may be the best therapeutic target. Thus, some monoclonal antibodies directed against Aβ target primarily Aβ plaques (donanemab, aducanumab, gantenerumab > lecanemab), while others target primarily Aβ oligomers (ACU193 > crenezumab, aducanumab), or Aβ protofibrils (lecanemab). See Figure X below, “Aβ/amyloid pathways.”
While methods other than PET Aβ for identifying pathologic brain amyloid exist (cerebral spinal fluid [CSF] analysis, which requires a lumbar puncture), or may be emerging (simple blood tests), currently PET Aβ is the most commonly-used method for patient selection in trials for any AD therapy (CMS NCD 200.3) as well as patent selection for eligibility for new FDA-approved antiamyloid drugs, such as lecanemab. The purpose of using this test for patients is not to ensure an “accurate diagnosis” of AD for any given patient; moreover, excluding or diagnosing other diseases, some of which may be treatable and reversible, is standard in the clinical work up for AD, and the emergence of PET Aβ scans has not changed this (McKhann 2011, CMS 2013, Dubois 2021). Rather, biomarker positivity for patient selection increases the likelihood that the population overall would have a higher proportion of patients who truly have AD, than would occur if selection for antiamyloid monoclonal antibodies or other AD treatments were based on clinical assessment alone. In addition, with the recent development of treatments directed against amyloid, the PET scan would help to confirm the presence of brain amyloid, to alter the course of treatment for the patient and to demonstrate treatment results. In addition, the PET AB can be used to better select patients for AD trials such antiamyloid treatment trials. One would not expect a therapy that removes brain amyloid in Alzheimer’s patients to work in patients who do not actually have brain amyloid and the disease in the first place.
PET Aβ imaging
PET Aβ imaging is a minimally invasive diagnostic imaging procedure used to evaluate normal as well as diseased tissues in conditions such as cancer, ischemic heart disease and some neurologic disorders. Certain radioactive tracers allow for imaging of Aβ plaques in the brain, primarily aggregated, “neuritic plaques.” As discussed above, these plaques are the specific targets of some (but not all) contemporary antiamyloid mAb therapies in completed and ongoing Phase 3 trials designed for FDA traditional approval.
Evaluation of PET
In the Federal Registry publication, “Reconciling National Coverage Determinations on Positron Emission Tomography (PET) Neuroimaging for Dementia (at
https://www.federalregister.gov/documents/2018/04/11/2018-07410/medicare-program-reconciling-national-coverage-determinations-on-positron-emission-tomography-pet), we noted that “[s]pecifically for diagnostic imaging tests, the overall assessment focuses on whether use of the test to guide patient management and treatment improves health outcomes (also referred to as clinical utility).” We also stated that “[b]efore appropriately reaching a consideration of outcomes, two fundamental properties of diagnostic tests need to be established: (1) the test accurately and reliably measures the intended analyte, factor, or component (also referred to as analytic validity); and (2) the test accurately and reliably identifies the condition or disorder of interest (also referred to as clinical validity).” But these “two fundamental properties” are stepping stones toward demonstration of clinical utility; for what matters ultimately is patient health outcomes.
III. History of Medicare Coverage
As of September 27, 2013, CMS has provided coverage for PET Aß imaging under CED. The September 27, 2013 amyloid PET NCD resulted in non-coverage of amyloid PET for dementia and neurodegenerative disease under § 1862(a)(1)(A); however, coverage was made available in the context of clinical studies. There, one amyloid PET scan per patient would be covered through coverage with evidence development (CED) pursuant to section 1862(a)(1)(E) of the Act (NCD 200.6.20 at https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=356&ncdver=1). The diagnostic test is covered under certain research parameters "in two scenarios: (1) To exclude Alzheimer’s disease (AD) in narrowly defined and clinically difficult differential diagnoses, such as AD versus frontotemporal dementia (FTD); and (2) to enrich clinical trials seeking better treatments or prevention strategies, by allowing for selection of patients on the basis of biological as well as clinical and epidemiological factors" (CMS 2013, 4). Prior to September 27, 2013, CMS did not previously cover PET Aβ imaging.
A. Current Request
CMS internally generated the opening of this NCD analysis based on stakeholder feedback, including public comments received during the finalization of NCD 200.3 (Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease, available at https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305 ) because it is anticipated that clinical study protocols may involve more than one PET Aß scan per patient.
B. Benefit Category
Medicare is a defined benefit program. For an item or service to be covered by the Medicare program, it must fall within one of the statutorily defined benefit categories outlined in the Social Security Act.
PET is considered to be within the following benefit category: other diagnostic tests §1861(s)(3) of the Act. Among other things, diagnostic tests must produce results that the ordering physician can use in the management of a beneficiary’s specific medical condition. 42 C.F.R. § 410.32.
IV. Timeline of Recent Activities
| Date | Actions Taken |
|---|
June 16, 2022 |
CMS opens an NCA for Initial 30-day public comment period begins. |
| July 15, 2022 |
First public comment period ends. CMS receives 36 comments. |
| December 15, 2002 |
CMS did not issue a proposed NCD on Beta Amyloid Positron Emission Tomography in Dementia and Neurodegenerative Disease and said a proposed decision is forthcoming after CMS has reviewed newly published evidence that is relevant to the proposed NCD. |
| July 17, 2023 |
Proposed Decision Memorandum posted. 30-day public comment period begins. |
V. Food and Drug Administration (FDA) Status
The FDA has reviewed and approved three radiopharmaceuticals for PET Aβ imaging, 18F-florbetapir (Amyvid™), 18F-florbetaben (Neuraceq™) and 18F-flutemetamol (Vizamyl™), to estimate Aβ neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) and other causes of cognitive decline. The FDA-approved prescribing information (PI) for these products includes description of studies that included healthy adult subjects and subjects with a range of cognitive disorders including mild cognitive impairment (MCI), AD dementia, and other (non-AD) types of dementing disorders, as well as terminally ill patients with no cognitive impairment. The PIs state that a negative PET scan with these radiopharmaceuticals is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition and reduces the likelihood that a patient’s cognitive impairment is due to AD but does not preclude the development of brain amyloid in the future, that a positive scan indicates moderate to frequent amyloid neuritic plaques that are present in patients with AD but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition, and that a positive scan does not establish a diagnosis of AD or other cognitive disorder.
The FDA-approved PIs for these PET Aβ imaging products indicate that safety and effectiveness have not been established for predicting development of dementia or other neurologic condition or for monitoring response to therapies. Additionally, the PIs note that PET images obtained with these radiopharmaceuticals should be interpreted only by readers who successfully complete a special training program, which is provided by the manufacturers of each of these radiopharmaceuticals.
VI. General Methodological Principles
When making NCDs under section 1862(a)(1)(A), CMS generally evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service falling within a benefit category is reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member. The critical appraisal of the evidence enables us to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for beneficiaries. An improved health outcome is one of several considerations in determining whether an item or service is reasonable and necessary.
A detailed account of the methodological principles of study design that the Agency utilizes to assess the relevant literature on a therapeutic or diagnostic item or service for specific conditions can be found in Appendix A.
Public comments sometimes cite published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination. Public comments that contain personal health information (PHI) will be redacted to remove PHI or will not be made available to the public. CMS responds in detail to the public comments on a proposed national coverage determination when issuing the final national coverage determination.
VII. Evidence
A. Introduction
This section provides a summary of the evidence we considered during our review. The evidence reviewed to date includes the peer-reviewed published medical literature on clinical trials and major studies that aim to demonstrate that interventions guided by PET Aꞵ imaging lead to improved health outcomes for patients, including Medicare beneficiaries.
B. Discussion
1. Key Question
For this NCD reconsideration, CMS initially focused on the following question:
- Should CMS revise the current policy that allows coverage of one PET Aβ scan per patient through CED?
In response to the comments received during the initial 30-day public comment period requesting that CMS remove the CED requirement from the NCD, as well as new evidence that became available, CMS is addressing the question below:
- Should CMS remove the current NCD for beta amyloid PET in dementia and neurodegenerative disease?
2. External Technology Assessments
CMS did not request an external technology assessment (TA) on this issue.
3. Internal Technology Assessment
The literature search for this NCD reconsideration was two-tiered. An initial search built off the extensive search performed for the directly related treatment NCD on monoclonal antibodies directed against amyloid (published April 7, 2022) (https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305).
The data bases Academic Search Premier, CINAHL, Google Scholar, Ovid Medline, PubMed, Scopus, and Web of Science, for English language articles in peer-reviewed journals, published from 2010 – spring 2022, using the search terms ‘phase 3 clinical trials’, ‘beta-amyloid’, and ‘monoclonal antibodies’. To ensure capture of all relevant articles, the search was conducted independently and concurrently (for 2010 – 2021) by a NIH librarian, International Consulting Associates (ICA) (a third-party evidence-review contractor to CMS), and the CMS Coverage and Analysis Group (CAG). CAG searched for additional pivotal trials and other major studies that aimed to demonstrate that interventions guided by PET Aꞵ imaging lead to improved health outcomes for Medicare beneficiaries, through August 2022 (a subsequent search through December 2022 did not find additional amyloid PET clinical trials on AD health outcomes).
In addition to peer-reviewed articles, we reviewed reports from other agencies, including the FDA, the Guideline Development, Dissemination and Implementation Subcommittee of the American Academy of Neurology, the National Institute on Aging, and the National Institute for Health Care and Excellence.
Finally, CAG reviewed the pertinent, published medical literature cited by commenters in the first public comment period.
4. Medicare Evidence Development & Coverage Advisory Committee (MEDCAC)
No MEDCAC meeting was convened.
5. Evidence Table
Table 1. Key contemporary studies on beta-amyloid PET scans
| |
Population |
|
Yr published
(enrollment) |
Study / 1st Author |
Size, Criteria |
Age (yrs) |
Sex (% F) |
Race, Ethnic
(Interv./Control) |
Intervention v.
Comparator |
Outcome
(primary) |
Time (mos, duration) |
Safety outcomes |
Results
(primary) |
2022
(2019-21) |
Clarity AD / van Dyck |
N = 1795
MCI due to AD, or mild AD dementia *
Aß pos. by PET or CSF
Impaired episodic memory § |
Lecanemab: 71.4±7.9
Placebo: 71.0±7.8 |
Lecanemab:51.6%
Placebo:53.0% |
White: 76.3% / 77.4%
Black: 2.3% / 2.7%
Asian: 17.1% / 16.9%
Hisp.: 12.5% / 12.3% |
Lecanemab v. Placebo |
Change in score of CDR-SB† from baseline at 18 mos |
18 |
Symptomatic ARIA-E
Lecanemab: 2.8%
Placebo: 0 Symptomatic ARIA-H
Lecanemab: 0.7%
Placebo: 0.2% |
Lecanemab superior for primary outcome.
Lecanemab: 1.21 change
Placebo: 1.66 change (difference, -0.45; 95% CI, -0.67 to -0.23; P<0.001) |
2022
(2014-2019) |
APEx / Vidoni |
N = 117
Underactive or sedentary
No cognitive impairment
Elevated and sub-threshold cerebral amyloid by PET |
Aerobic exercise
71.2±4.8
Education control
72.2±5.3 |
Aerobic exercise 70.5%
Education control 61.5% |
White: 98.7/89.7%
Black: 1.3% / 10.3% |
Aerobic exercise v. Educational control |
Global amyloid burden, change from baseline |
12 |
Related to intervention: aerobic exercise 31 mild (e.g., joint pain resolving with exercise modification), 2 moderate (e.g. joint pain temporarily halting exercise), and 0 severe event
Education control: 0 in all categories |
No difference between exercise and education control. Aerobic exercise 0.01
Education control 0.01 (P=0.93) |
2018
(2015-16) |
de Wilde |
N = 507
Patients visiting a memory clinic
Or, MCI from a 2nd memory clinic |
Dementia: 66±8
MCI: 67±8
SCD £:61±8 |
Dementia: 42%
MCI: 36%
SCD: 38% |
|
Pre-PET v. Post-PET |
Changes in diagnosis, diagnostic confidence, treatment, and patient experience, pre- v. post-PET |
|
|
PET results (Pos or Neg) associated with changes in diagnosis and treatment, for all groups. |
| 2021 |
Trailblazer-Alz / Mintun |
N = 257
Early, symptomatic AD
Tau PET with specified range |
Donanemab: 75±6
Placebo: 75±5 |
Donanemab: 52%
Placebo: 52% |
White: 93% / 96% |
Donanemab v. Placebo |
Change in score of iADRS from baseline at 76wks |
76 weeks |
Symptomatic ARIA-E
Donanemab: 6.1%
Placebo: 0.8% Symptomatic ARIA-H (not reported) “Serious adverse event”
Donanemab: 17.6%
Placebo:17.6% |
Donanemab better for primary outcome.
Donanemab: 6.86 change
Placebo: 10.06 change
(difference, 3.20; 95% CI, 0.12 to 6.27; P=0.04) |
| 2019 |
IDEAS / Rabinovici |
N = 11409
MCI or dementia of uncertain etiology |
75 (70-79) IQR |
MCI: 49.6%
Dementia: 52.8% |
MCI
Black: 3.0%
White: 90%
Other: 7.1%
Hispanic: 3.0% Dementia
Black: 5.0%
White: 85%
Other: 10%
Hispanic: 5.4% |
Pre-PET v. Post-PET |
Change in management (=30% overall) |
|
|
Overall Change
MCI: 60.2%
Dementia: 63.5%
(P<.001) |
* Albert 2011, McKhann 2011.
§ At least 1 standard deviation below the age-adjusted mean in the Wechsler Memory Scale IV–Logical Memory II.
† Clinical Dementia Rating (CDR)–Sum of Boxes (CDR-SB).
£ Subjective Cognitive Decline (SCD).
Additional Evidence Tables
Since the studies on monoclonal antibodies directed against amyloid used amyloid PET as the diagnostic test, the evidence from NCD 200.3 would be applicable to this amyloid PET reconsideration as well in the consideration of clinical utility with new drug treatments. Please see the evidence tables in the Decision memo for Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease (at https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305).
CMS approved CED studies
Since the initial amyloid PET NCD 220.6.20 in 2013, CMS approved four CED studies (see: https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Amyloid-PET). Of these, three have been completed. One study (New IDEAS) has not been completed due to enrollment issues and is not active. CMS appreciates the researchers and patients that have participated in all of the CMS approved studies and encourages completion of the remaining approved CED study.
- Study Title: New IDEAS: Imaging Dementia-Evidence for Amyloid Scanning Study
Sponsor: American College of Radiology
ClinicalTrials.gov Number: NCT04426539
CMS Approval Date: 4/21/2020; reported to be inactive.
- Study Title: Cognitive Training and Practice Effects in MCI
Sponsor: University of Utah
ClinicalTrials.gov Number: NCT02301546
CMS Approval Date: 01/05/2016
Completion Date: February 28, 2020
Duff K, Ying J, Suhrie KR, Dalley BCA, Atkinson TJ, Porter SM, Dixon AM, Hammers DB, Wolinsky FD. Computerized Cognitive Training in Amnestic Mild Cognitive Impairment: A Randomized Clinical Trial. J Geriatr Psychiatry Neurol. 2022 May;35(3):400-409. doi: 10.1177/08919887211006472. Epub 2021 Mar 30.
Authors concluded: “Although the experimental cognitive training program did not improve outcomes in those with MCI, the short-term effects of the control group should not be dismissed, which may alter treatment recommendations for these patients.”
- Study Title: Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) Study
Sponsor: American College of Radiology Imaging Network
ClinicalTrials.gov Number: NCT02420756
IDEAS Study site: http://ideas-study.org/
CMS Approval Date: 03/03/2015
Completed: December, 2017
Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, Hendrix J, Hillner BE, Olson C, Lesman-Segev OH, Romanoff J, Siegel BA, Whitmer RA, Carrillo MC. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA. 2019 Apr 2;321(13):1286-1294. doi: 10.1001/jama.2019.2000.
Authors concluded: “Among Medicare beneficiaries with MCI or dementia of uncertain etiology evaluated by dementia specialists, the use of amyloid PET was associated with changes in clinical management within 90 days. Further research is needed to determine whether amyloid PET is associated with improved clinical outcomes.”
Wilkins CH, Windon CC, Dilworth-Anderson P, Romanoff J, Gatsonis C, Hanna L, Apgar C, Gareen IF, Hill CV, Hillner BE, March A, Siegel BA, Whitmer RA, Carrillo MC, Rabinovici GD. Racial and Ethnic Differences in Amyloid PET Positivity in Individuals With Mild Cognitive Impairment or Dementia: A Secondary Analysis of the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) Cohort Study. JAMA Neurol. 2022 Oct 3;79(11):1139–47. doi: 10.1001/jamaneurol.2022.3157. Epub ahead of print. PMID: 36190710; PMCID: PMC9531087.
Authors concluded: “Racial and ethnic differences found in amyloid PET positivity among individuals with MCI and dementia in this study may indicate differences in underlying etiology of cognitive impairment and guide future treatment and prevention approaches.”
- Study Title: Effect of Aerobic Exercise on Pathophysiology in Preclinical Alzheimer’s Disease
Sponsor: National Institute on Aging
ClinicalTrials.gov Number: NCT02000583
CMS Approval Date: 04/02/2014,br>[Completed?]: December 31, 2019
Vidoni ED, Morris JK, Watts A, Perry M, Clutton J, Van Sciver A, Kamat AS, Mahnken J, Hunt SL, Townley R, Honea R, Shaw AR, Johnson DK, Vacek J, Burns JM. Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer's: A 1-year randomized controlled trial. PLoS One. 2021 Jan 14;16(1):e0244893. doi: 10.1371/journal.pone.0244893. eCollection 2021.
Authors concluded: “Aerobic exercise was not associated with reduced amyloid accumulation in cognitively normal older adults with cerebral amyloid. In spite of strong systemic cardiorespiratory effects of the intervention, the observed lack of cognitive or brain structure benefits suggests brain benefits of exercise reported in other studies are likely to be related to non-amyloid effects.”
VIII. Public Comment
Public comments sometimes cite the published clinical evidence and give CMS useful information. Public comments that give information on unpublished evidence such as the results of individual practitioners or patients are less rigorous and therefore less useful for making a coverage determination.
CMS uses the initial public comments to inform its proposed decision. CMS responds in detail to the public comments on a proposed decision when issuing the final decision memorandum. All comments that were submitted without personal health information may be viewed in their entirety by using the following link
Initial Comment Period: 06/16/2022 – 07/15/2022
During the 30-day comment period following the release of the tracking sheet, CMS received 36 comments. The majority of comments expressed that the scope of the reconsideration should be broader than the question of whether the current policy of one scan per patient should be revised, suggesting that the evidence is sufficient to end CED and provide national coverage of PET Aβ imaging, or have coverage determined by Medicare administrative contractors (MACs). Most of these commenters also believed that the current limit of one PET Aβ scan per patient should be removed if CED were to remain. A few commenters also suggested that there should not be coverage for PET Aβ scans because clinical utility had not been established.
The comments included seven from physicians, and eight from medical companies. We also received 17 comments on behalf of national associations/professional societies, including the American Academy of Neurology (AAN), American College of Radiology (ACR), American Geriatrics Society (AGS), Alliance for Aging Research, Alliance for Patient Access (AfPA), Alzheimer’s Association, Biotechnology Innovation Organization (BIO), Caregiver Action Network, Leaders Engaged on Alzheimer’s Disease (LEAD), Medical Imaging & Technology Alliance (MITA), National Center for Health Research (NCHR), National Down Syndrome Society (NDSS), Partnership to Fight Chronic Disease (Pharmaceutical Research and Manufacturers of America (PhRMA), Society for Women’s Health Research (SWHR), Society of Nuclear Medicine and Molecular Imaging (SNMMI), UsAgainstAlzheimer’s.
IX. CMS Analysis
Introduction
NCDs are determinations by the Secretary with respect to whether or not a particular item or service is covered nationally by Medicare (§1869(f)(1)(B) of the Act). In order to be covered by Medicare, an item or service must fall within one or more benefit categories contained within Part A or Part B, and must not be otherwise excluded from coverage. Moreover, with limited exceptions, the expenses incurred for items or services must be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member (§1862(a)(1)(A) of the Act).
CMS is not required to establish an NCD. The Supreme Court has recognized that “[t]he Secretary's decision as to whether a particular medical service is ‘reasonable and necessary’ and the means by which she implements her decision, whether by promulgating a generally applicable rule or by allowing individual adjudication, are clearly discretionary decisions.” Heckler v. Ringer, 466 U.S. 602, 617 (1984). See also, Almy v. Sebelius, 679 F.3d 297, 303-04 (4th Cir. 2012) (“The Medicare statute preserves this discretion for the Secretary, leaving it to her judgment whether to proceed by implementing an NCD, by allowing regional contractors to adopt an LCD, or by deciding individual cases through the adjudicative process.”); International Rehabilitative Services Inc. v. Sebelius, 688 F.3d 994, 1001 (9th Cir. 2012) (“But while the agency may make coverage determinations via up-front rules, it is not required to do so; rather, the agency has discretion in whether to make coverage determinations by up-front rulemaking or by case-by-case adjudication.”)
The 2013 NCD for amyloid PET scans (220.6.20) notes that the available evidence suggested these scans were promising and therefore supported further research under §1862(a)(1)(E) of the Social Security Act (the Act) through CED. While there have been CED studies approved early when the NCD was established, there have been no CED study approved since 2016 (note: the New IDEAS study was a limited extension of the 2015 IDEAS study for underserved populations and is not active). Based on published articles and conversations with stakeholders, we have been informed that a number of relevant clinical studies have been conducted and new evidence has been developed outside the context of the CMS CED, specifically for development of new treatments directed against amyloid (see CMS NCD 200.3 (at https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=305)). The research on amyloid PET has advanced and has been redirected towards antiamyloid treatments (e.g., Clarity AD trial) and the coverage of separate CED studies are no longer needed. With the new developments in medical care and treatments, the use of amyloid PET has changed and has become more individualized with treatments. Based on stakeholder feedback, we do not anticipate that any new CED protocols will be submitted for review under this NCD (we have not received any new protocol since January 2016).
For this NCD reconsideration, CMS initially focused on the following question:
- Should CMS revise the current policy of one PET Aβ scan per patient through CED?
In response to the comments received during the initial 30-day public comment period requesting that CMS remove the CED requirement from the NCD, as well as new evidence that became available, CMS is addressing the question below:
- Should CMS remove the current NCD for beta amyloid PET in dementia and neurodegenerative disease?
The clinical research is currently focused on new treatments directed against amyloid which require confirmation of the presence of brain amyloid using tests such as amyloid PET.
NCD Removal
Since the establishment of the amyloid PET NCD in 2013, there have been a number of advances in medical care and treatments for AD. For example, addressing certain health risk factors and comorbidities such as high blood pressure has been emphasized in overall treatment. The 2020 Lancet Commission concludes that lifestyle changes and treatment of 12 modifiable risk factors associated with Alzheimer’s disease could potentially prevent or delay up to 40% of dementia cases (Livingston 2020). Standards of care for patients with AD have also advanced. For example, the American Academy of Neurology (AAN) recently published a review of the current evidence and clinical practice guidelines (Day et al., 2022). The development of antiamyloid drug treatments, such as monoclonal antibodies, laboratory-made proteins designed to bind a specific substance in the body, with the goal of marking it for destruction by the body’s immune system, is particularly promising (CMS NCD 200.3, 2022; available at https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=375&ncdver=1&keywordtype=starts&keyword=monoclonal&bc=0.)
The advances in medical care and standards of practice and development of proven treatments have altered the application of amyloid PET scans in clinical practice and research. For example, promising antiamyloid drug treatments to improve health outcomes such as cognition and function would not be expected to have a clinical impact in the absence of brain amyloid. Appropriate patient selection is key to ensuring benefits outweigh harms of newly developed drugs targeting amyloid. By including useful diagnostic tests that can detect beta amyloid on the brain, harms of antiamyloid treatments would be avoided because the drug would not be given to patients that do not have brain amyloid and potentially stopped when brain amyloid was completely removed. When the 2013 CED questions were created, advances in the application of amyloid PET scans and the importance of patient selection for promising treatments were not known. Currently, amyloid PET scans can be used to confirm presence of brain amyloid to select appropriate patients for proven antiamyloid treatments depending on individual patient characteristics. Advancements in anitamyloid treatments for AD are accelerating. Isolated PET research separate from treatment trials through this CED NCD is no longer needed and no longer being requested by stakeholders. Moreover, stakeholders and patients have specifically noted that the once in a lifetime limit on amyloid PET is outdated and not clinically appropriate due to the development of antiamyloid treatments and the need to confirm the presence of amyloid to start these treatments and to possibly discontinue treatments when brain amyloid has been completely removed to avoid unnecessary treatment harms.
Recent treatment trials requiring biomarker evidence of amyloid pathology for patient enrollment generally have used PET Aꞵ brain scans. The importance of this requirement is exemplified by early, failed solanezumab trials. An analysis of secondary outcomes reported that roughly 25% of trial participants did not have positive brain amyloid on PET scans and may never have had AD (Siemers 2016). A treatment aimed at improving cognition and function for AD patients, precisely because it modifies
underlying AD pathophysiology, is not likely to succeed if it is not AD from brain amyloid that is causing the patients’ symptoms in the first place. Other types of treatments that are not classified as monoclonal antibodies are in accelerated development, as well, such as inhibitor drugs and nanotechnology. These innovative treatments were not available in 2013 when the existing coverage criteria and limitation were established and would also need confirmation of the presence of brain amyloid for similar reasons. We believe that allowing local MAC discretion to make a coverage decision better serves the needs of the Medicare program and its beneficiaries at this time.
Individual patient factors need to be considered for drug treatments such as the type and number of medical comorbidities a patient may have and the respective concomitant medication treatments. Certain medications such anticoagulants may affect the benefits and harms of certain antiamyloid drugs. In addition to the above, the use of amyloid PET would be dependent on the specific anti-amyloid treatment, individual patient response (amount of brain amyloid reduction), adverse events and corresponding need for subsequent testing to potentially stop treatments. For example, certain drugs may remove brain amyloid at different rates in different patients. For patients that have brain amyloid reduced completely as shown on a test such as a PET scan, a physician and patient may consider the benefits and harms of certain drugs and whether discontinuation of treatment would be appropriate.
The MACs may also take into consideration the local clinical environment and institutional factors in making coverage determinations. As one example, Grand and colleagues (2011) reported:
"The best approach to the care of individuals affected by dementia includes support from multiple sources. These may be integrated, parallel, or a combination of both. It is ideal for patients and caregivers to seek a blend of multidisciplinary services that come from various healthcare providers, social service agencies, and professionals from outside the field of healthcare. Multidisciplinary teams vary in their composition and may be structured either formally or informally. Team construction is usually dictated by resources, including time, availability, finances, and geographic location. Regardless of structure, successful teams are characterized by a shared commitment to quality care and an appreciation for the contributions of each team member.
Multidisciplinary teams involved in dementia care tend to be based primarily on the availability of service resources, in addition to the social and cultural context of the community. Team members often include neurologists, geriatricians, neuropsychologists, nurse practitioners, physical/occupational therapists, nutritionists, and social workers."
We believe removal of NCD 220.6.20 will allow appropriate coverage of amyloid PET scans and will greatly reduce provider and patient burden from the existing requirements and test limitation. Stakeholders and patients have particularly emphasized the constraints on choice of treatments and appropriate management of proven antiamyloid treatments due to the once in a lifetime limitation. As new treatments directed against amyloid for patients with AD, whether monoclonal antibodies, inhibitor drugs, nanotechnology or other new technology, are developed and approved by the FDA, the MACs are able to promptly respond to the evidence on proven treatments for individual patients. Thus, for these reasons, we propose to remove the NCD PET beta amyloid imaging, which would allow local MACs to make coverage determinations regarding the use of Aꞵ PET imaging, to include, covering more than one scan per patient’s lifetime and use within or outside the context of a CMS approved study.
Health Disparities
Significant differences in the prevalence of AD across racial and ethnic groups have previously been reported. Research has shown that AD is more prevalent in Blacks and Hispanics when compared to Whites, with Blacks being up to two times more likely to have AD and other dementias when compared to Whites, and Hispanics being about one and a half times more likely than Whites to have AD and other dementias (AA 2020, Mayeda 2016). Despite the higher prevalence of AD and other dementias in Blacks and Hispanics, they are less likely to have a diagnosis when compared to Whites. In a recent survey by the Alzheimer’s Association (2021), it was found that Black, Hispanic, and Native Americans were twice as likely than Whites to say they would not see a doctor if experiencing thinking or memory problems. This can partially be explained by differing racial and ethnic beliefs about AD. Significantly more Black and non-White Americans believe that symptoms of AD, such as significant memory loss, are part of the normal aging process when compared to White Americans (AA 2021, Connell 2009). This may also be partially due to higher rates of discrimination when seeking dementia-related health care. Recent surveys reveal that Blacks reported the highest level of discrimination in dementia health care (50%), followed by Native Americans (42%), Asians (34%), and Hispanics (33%), compared to Non-Hispanic Whites (9%) (AA 2021).
Additionally, under-representation of members of racial and ethnic groups in research relating to AD further exacerbates disparities in our understanding of, and ability to provide appropriate health care for, all individuals CMS serves related to these conditions. Some barriers to the recruitment of under-represented patients can include language, logistical barriers (e.g., time, travel), and a long-standing mistrust of the medical establishment (Watson 2014). In the same survey previously mentioned by the Alzheimer’s Association (2021), 62% of Black Americans believed that medical research is biased against people of color, and Black Americans expressed less interest in participating in clinical trials for AD than any other group surveyed (White and non-White). Additionally, only 53% of Black Americans surveyed believed that a future cure for AD would be equally shared regardless of race and ethnicity.
IX. Conclusion
CMS is proposing to remove the NCD at § 220.6.20, ending CED for PET beta amyloid imaging and permitting Medicare coverage determinations for PET beta amyloid imaging to be made by MACs under § 1862(a)(1)(A) of the Act.
APPENDIX A
General Methodological Principles of Study
Design
(Section VI of the Decision Memorandum)
When making national coverage determinations, CMS evaluates relevant clinical evidence to determine whether or not the evidence is of sufficient quality to support a finding that an item or service is reasonable and necessary. The overall objective for the critical appraisal of the evidence is to determine to what degree we are confident that: 1) the specific assessment questions can be answered conclusively; and 2) the intervention will improve health outcomes for patients.
We divide the assessment of clinical evidence into three stages: 1) the quality of the individual studies; 2) the generalizability of findings from individual studies to the Medicare population; and 3) overarching conclusions that can be drawn from the body of the evidence on the direction and magnitude of the intervention’s potential risks and benefits.
The methodological principles described below represent a broad discussion of the issues we consider when reviewing clinical evidence. However, it should be noted that each coverage determination has its unique methodological aspects.
Assessing Individual Studies
Methodologists have developed criteria to determine weaknesses and strengths of clinical research. Strength of evidence generally refers to: 1) the scientific validity underlying study findings regarding causal relationships between health care interventions and health outcomes; and 2) the reduction of bias. In general, some of the methodological attributes associated with stronger evidence include those listed below:
- Use of randomization (allocation of patients to either intervention or control group) in order to minimize bias.
- Use of contemporaneous control groups (rather than historical controls) in order to ensure comparability between the intervention and control groups.
- Prospective (rather than retrospective) studies to ensure a more thorough and systematical assessment of factors related to outcomes.
- Larger sample sizes in studies to demonstrate both statistically significant as well as clinically significant outcomes that can be extrapolated to the Medicare population. Sample size should be large enough to make chance an unlikely explanation for what was found.
- Masking (blinding) to ensure patients and investigators do not know to that group patients were assigned (intervention or control). This is important especially in subjective outcomes, such as pain or quality of life, where enthusiasm and psychological factors may lead to an improved perceived outcome by either the patient or assessor.
Regardless of whether the design of a study is a randomized controlled trial, a non-randomized controlled trial, a cohort study or a case-control study, the primary criterion for methodological strength or quality is to the extent that differences between intervention and control groups can be attributed to the intervention studied. This is known as internal validity. Various types of bias can undermine internal validity. These include:
- Different characteristics between patients participating and those theoretically eligible for study but not participating (selection bias).
- Co-interventions or provision of care apart from the intervention under evaluation (performance bias).
- Differential assessment of outcome (detection bias).
- Occurrence and reporting of patients who do not complete the study (attrition bias).
In principle, rankings of research design have been based on the ability of each study design category to minimize these biases. A randomized controlled trial minimizes systematic bias (in theory) by selecting a sample of participants from a particular population and allocating them randomly to the intervention and control groups. Thus, in general, randomized controlled studies have been typically assigned the greatest strength, followed by non-randomized clinical trials and controlled observational studies. The design, conduct and analysis of trials are important factors as well. For example, a well-designed and conducted observational study with a large sample size may provide stronger evidence than a poorly designed and conducted randomized controlled trial with a small sample size. The following is a representative list of study designs (some of that have alternative names) ranked from most to least methodologically rigorous in their potential ability to minimize systematic bias:
Randomized controlled trials
Non-randomized controlled trials
Prospective cohort studies
Retrospective case control studies
Cross-sectional studies
Surveillance studies (e. g. , using registries or
surveys)
Consecutive case series
Single case reports
When there are merely associations but not causal relationships between a study’s variables and outcomes, it is important not to draw causal inferences. Confounding refers to independent variables that systematically vary with the causal variable. This distorts measurement of the outcome of interest because its effect size is mixed with the effects of other extraneous factors. For observational, and in some cases randomized controlled trials, the method in that confounding factors are handled (either through stratification or appropriate statistical modeling) are of particular concern. For example, in order to interpret and generalize conclusions to our population of Medicare patients, it may be necessary for studies to match or stratify their intervention and control groups by patient age or co-morbidities.
Methodological strength is, therefore, a multidimensional concept that relates to the design, implementation and analysis of a clinical study. In addition, thorough documentation of the conduct of the research, particularly study selection criteria, rate of attrition and process for data collection, is essential for CMS to adequately assess and consider the evidence.
Generalizability of Clinical Evidence to the Medicare Population
The applicability of the results of a study to other populations, settings, treatment regimens and outcomes assessed is known as external validity. Even well-designed and well-conducted trials may not supply the evidence needed if the results of a study are not applicable to the Medicare population. Evidence that provides accurate information about a population or setting not well represented in the Medicare program would be considered but would suffer from limited generalizability.
The extent to that the results of a trial are applicable to other circumstances is often a matter of judgment that depends on specific study characteristics, primarily the patient population studied (age, sex, severity of disease and presence of co-morbidities) and the care setting (primary to tertiary level of care, as well as the experience and specialization of the care provider). Additional relevant variables are treatment regimens (dosage, timing and route of administration), co-interventions or concomitant therapies, and type of outcome and length of follow-up.
The level of care and the experience of the providers in the study are other crucial elements in assessing a study’s external validity. Trial participants in an academic medical center may receive more or different attention than is typically available in non-tertiary settings. For example, an investigator’s lengthy and detailed explanations of the potential benefits of the intervention and/or the use of new equipment provided to the academic center by the study sponsor may raise doubts about the applicability of study findings to community practice.
Given the evidence available in the research literature, some degree of generalization about an intervention’s potential benefits and harms is invariably required in making coverage determinations for the Medicare population. Conditions that assist us in making reasonable generalizations are biologic plausibility, similarities between the populations studied and Medicare patients (age, sex, ethnicity and clinical presentation) and similarities of the intervention studied to those that would be routinely available in community practice.
A study’s selected outcomes are an important consideration in generalizing available clinical evidence to Medicare coverage determinations. One of the goals of our determination process is to assess health outcomes. These outcomes include resultant risks and benefits such as increased or decreased morbidity and mortality. In order to make this determination, it is often necessary to evaluate whether the strength of the evidence is adequate to draw conclusions about the direction and magnitude of each individual outcome relevant to the intervention under study. In addition, it is important that an intervention’s benefits are clinically significant and durable, rather than marginal or short-lived. Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits.
If key health outcomes have not been studied or the direction of clinical effect is inconclusive, we may also evaluate the strength and adequacy of indirect evidence linking intermediate or surrogate outcomes to our outcomes of interest.
Assessing the Relative Magnitude of Risks and Benefits
Generally, an intervention is not reasonable and necessary if its risks outweigh its benefits. Health outcomes are one of several considerations in determining whether an item or service is reasonable and necessary. CMS places greater emphasis on health outcomes actually experienced by patients, such as quality of life, functional status, duration of disability, morbidity and mortality, and less emphasis on outcomes that patients do not directly experience, such as intermediate outcomes, surrogate outcomes, and laboratory or radiographic responses. The direction, magnitude, and consistency of the risks and benefits across studies are also important considerations. Based on the analysis of the strength of the evidence, CMS assesses the relative magnitude of an intervention or technology’s benefits and risk of harm to Medicare beneficiaries.


