To: CAG File #00040N
From: Richard P. Coyne
Deputy Director, Coverage and Analysis
John J. Whyte, MD, MPH
Julie K. Taitsman, MD, JD
Medical Officers, Coverage and Analysis
Subject: Percutaneous Image-Guided Breast Biopsy
Date: December 7, 1999
This memo serves five purposes: (1) outlines the epidemiology of breast cancer, with particular emphasis on the impact of the disease in the elderly population; (2) briefly describes the available methods of diagnosing breast cancer; (3) reviews the history of Medicare’s coverage policies regarding percutaneous image-guided breast biopsies; (4) analyzes the scientific literature relating to the various methods of performing breast biopsies; (5) explains the rationale for a new national coverage policy regarding percutaneous image-guided breast biopsy.
Epidemiology
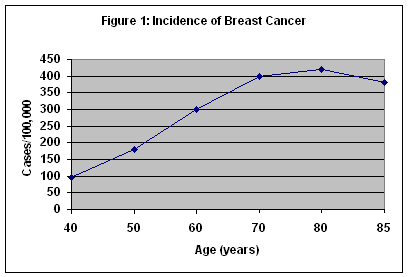
Breast cancer is the most common form of cancer in women in this country, and the second leading cause of cancer death. It is the most common cause of cancer deaths in women over 65 years of age. The incidence of breast cancer increases up to 80 years of age and plateaus between 80 and 85 years. (Figure 1) Although breast cancer is often perceived as a disease of middle-aged women, it is important to note that more than 50 percent of breast cancers occur in women 65 years of age and older. Because of increased comorbidities, the death rate for breast cancer in women over 65 years of age is nearly 50 percent. For all ages, over 40,000 women die from breast cancer each year. It is estimated that an American woman’s lifetime risk of developing breast cancer is about one in eight.
Detection of Breast Cancer
A. Screening
Historically, most breast cancers presented as an asymptomatic palpable breast lump. As the impact of breast cancer drew greater attention and treatment methods developed, the importance of early detection became apparent. Early detection relies on screening protocols involving physical examination of the breast by both the patient and the clinician, as well as imaging studies which may detect nonpalpable lesions. Imaging primarily implies screening mammography although screening ultrasound is employed in some situations.1 The American Cancer Society and the National Cancer Institute currently recommend that women over 40 years old undergo screening mammograms on an annual basis. Slightly fewer than half of the 90 million women over forty years old undergo such screening. Although Medicare generally denies coverage for preventive services, statutory language in the Balance Budget Act of 1997 authorizes Medicare payments for yearly mammography of female beneficiaries over age 40 years.2 Currently, the increased practice of screening mammography has made an impact such that more than half of newly diagnosed breast cancers are detected when they are smaller than 2 cm. It is estimated that 5 cancers are detected for every 1000 women screened.
The American College of Radiology (ACR) employs a system for reporting mammography results. This Breast Imaging Reporting and Data System (BIRADS) provides a standardized lexicon with which radiologists may report their interpretation of a mammogram (Table 1). The radiologist may also convey information regarding the composition of the breast tissue by means of another BIRADS classification scheme (Table 2).
Table 1: BIRADS Grading of Mammograms
Grade I Negative
Grade II Benign finding
Grade III Probably benign
Grade IV Suspicious abnormality
Grade V Highly suggestive of malignant neoplasm
Table 2: BIRADS Classification of Breast Tissue Composition
Class I Fatty tissue
Class II Scattered fibroglandular densities
Class III Heterogeneously dense breast tissue
Class IV Extremely dense breast tissue
B. Diagnosis by Breast Biopsy
A radiographic abnormality may raise the suspicion of breast cancer but it does not provide a definitive diagnosis; confirmation by histopathology of the breast tissue is always required.3 Depending on the level of suspicion of the radiographic abnormality, the physician may advise the patient to follow the lesion radiographically, or to undergo a breast biopsy to obtain a specimen to be sent for histologic diagnosis. There are approximately one million breast biopsies performed in the United States each year. About 40% of these biopsies are performed to evaluate nonpalpable lesions. For 70-80% of all breast biopsies, the pathology report proves benign. For cancerous lesions, the breakdown of histologic diagnoses is as follows: 80% ductal carcinoma, 10% lobular carcinoma, 5% medullary carcinoma, and 5% other histology. The most common histologic diagnoses are listed in Table 3.
Table 3: Histologic Diagnoses of Biopsied Breast Tissue
- Normal Breast Tissue
- Fibrocystic Change: A common finding involving morphologic changes which usually have no clinical significance
- Fibroadenoma: The most common benign breast tumor
- Atypical Ductal Hyperplasia (ADH): A noncancerous abnormality which causes an increased risk of developing carcinoma
- Lobular Carcinoma In Situ (LCIS): A proliferative breast lesion arising in the terminal ducts or ductules which causes a greatly increased risk of developing infiltrating carcinomas
- Ductal Carcinoma In Situ (DCIS): A proliferative breast lesion arising in the ducts which causes a greatly increased risk of developing infiltrating carcinomas
- Infiltrating Ductal Carcinoma (IDC): Accounts for about 80% of invasive breast cancers
- Infiltrating Lobular Carcinoma: Accounts for about 10% of invasive breast cancers
- Medullary Carcinoma: Accounts for about 5% of invasive breast cancers
C. Methods of Breast Biopsy
Currently, the vast majority of breast biopsies performed are open surgical biopsies. Open surgical biopsies (OSB) are performed in an operating theater, usually following needle wire localization placed in the radiology suite. Open surgical biopsy has historically been accepted as the gold standard method of obtaining a specimen of breast tissue for histologic diagnosis. Typically, 5-25 gm of tissue is removed through a surgical incision site 3-6 cm in length. This large amount of tissue allows accuracy of the diagnosis, and at times can even be therapeutic. It is important to note, however, that it is an imperfect gold standard, with an error rate estimated between 0.2 to as high as 20%.4 Sources of error include incision error, localization error (e.g. dependent upon use of a wire), and tissue selection error. Other drawbacks include the fact that the technique can be disfiguring, time consuming, and expensive (both direct and indirect costs).
Less invasive biopsy methods, however, are gradually increasing in use, often replacing surgical biopsy as the next diagnostic step for some radiographically detected lesions. Several of these methods employ image guidance systems to obtain biopsy specimens through a percutaneous incision. The image guidance may be provided by ultrasound or stereotactic (two mammographic views) imaging, both of which help the clinician more accurately target the area of breast tissue which produced an abnormality on the initial imaging study. Fine Needle Aspiration (FNA), another method of biopsy, is used infrequently due to its low accuracy. The false negative rare is roughly 30%, and insufficient tissue is obtained in 45% of procedures. Of particular note, FNA diagnoses are based on cytology – as a result, ductal carcinoma in situ and infiltrating ductal cancer cannot be distinguished by FNA. The percentage of breast biopsies performed by these methods are listed in Table 4.
Table 4: Percentage of Breast Biopsies Performed by Particular Methods
Open Surgical Biopsy: 78%
Stereotactic-Guided Biopsy: 14%
Ultrasound-Guided Biopsy: 7%
Fine Needle Aspiration (FNA): 1%
D. Percutaneous Image-Guided Breast Biopsy
There are various methods of percutaneous image-guided breast biopsy including: (1) Directional, Vacuum Assisted Biopsy Performed with Imaging Guidance (DVAB) (2) Automated Surgical Biopsy (3) Needle Core Biopsy
1. Directional, Vacuum Assisted Biopsy Performed with Imaging Guidance (DVAB)
Image guided, directional vacuum assisted systems offer a method of obtaining breast tissue for histologic analysis by a less invasive means than with open surgical excision. The patient typically lies prone on a table equipped with an imaging system with her breast protruding through a hole in the table.5 After administration of local anesthesia, the physician (radiologist or surgeon) makes a 3-5mm skin incision through which the probe is inserted. Employing image guidance (either stereotactic mammography or ultrasound), the probe is advanced to the tissue of mammographic concern. The vacuum draws tissue samples into the probe which are then cut, collected, and sent for pathology analysis. The amount of tissue removed is approximately 100 mg per core. Numerous tissue samples may be collected during the procedure. If an 11-gauge device is used, the clinician has the option of placing a radiopaque metal clip through the probe, to assist with future identification of the biopsy site. For biopsies performed to investigate microcalcifications, the tissue sample is radiographed to ensure presence of calcifications in the specimen.
Currently two companies market vacuum assisted biopsy devices. The Mammotome device is manufactured by Biopsys Medical, Inc., which is owned by Ethicon Endo-Surgery, Inc., of Cincinnati, Ohio, a Johnson & Johnson company. 11- and 14-gauge versions of the Mammotome device are available. A handheld Mammotome was approved by the FDA in September, 1999 and is presently on the market. The Minimally Invasive Breast Biopsy (MIBB) system is manufactured by US Surgical, a subsidiary of Tyco.
2. Automated Surgical Biopsy
The Advanced Breast Biopsy Instrument (ABBI) is one device used in automated surgical biopsies. The patient lies prone on a stereotactic table and the breast is compressed. The ABBI is deployed from the site offering the shortest distance from skin to lesion. After injection of local anesthetic agents, a 1-3 cm incision is made in the breast through which an open-ended cannula (10, 15, or 20 mm in diameter) containing a motorized oscillating blade is inserted. The cannula is advanced into the breast under stereotactic mammographic guidance (ultrasound guidance is not compatible with this technique). A cylinder of breast tissue is cut by an oscillating blade and then a wire loop cautery. The device is then removed from the breast and the detached plug of tissue is then extracted from the cannula. The tissue removed is a core of tissue from the skin to the abnormality. Radiographic examination of the specimen is then performed. If necessary, additional tissues are removed via the tract left at extraction of the cannula. If the entire mammographic abnormality is determined to have been removed, the patient is repositioned supinely and the skin incision is then closed with sutures. One tissue sample is obtained during a procedure employing the ABBI cannula. ABBI procedures are usually performed with local anesthesia, however, occasionally conscious sedation can be employed for extremely anxious patients.
Currently, ABBI is the only automated surgical biopsy device. The ABBI is a joint project of United States Surgical Corporation, Norwalk, Connecticut, and Lorad, Danbury, Connecticut.
3. Needle Core Biopsy with Image Guidance
Needle core biopsy involves deployment of a large core needle into the mammographically suspicious breast tissue by means of an automated gun. Image guidance can be either stereotactic or ultrasound. (Ultrasound is the predominant technique.) The patient lies prone on a table equipped with an imaging system with her breast protruding through an aperture in the table.6 After administration of local anesthesia, the physician (radiologist or surgeon) makes a skin incision, usually smaller than one quarter of an inch. A hollow needle is then inserted through this incision and directed towards the lesion employing imaging-guidance. When the needle is deemed to be in the proper position, the automated gun is fired to obtain a tissue sample. Numerous tissue samples (typically 10-20) may be obtained during a procedure. Total tissue removed is typically 10-15 mg per core.7 This technique is very physician-dependent, requiring pinpoint accuracy, since tissue is acquired only in the direct line of fire. For biopsies performed to investigate microcalcifications, the tissue sample is radiographed to ensure presence of calcifications in the specimen.
There are several brands of needles and guns. The Tru-Cut and Ultra-Core biopsy needles are manufactured by Ultracore Medical Device Technologies of Gainesville, Florida. The 14-gauge Manan cutting needle is manufactured by Manan Medical Products, Northbrook, Illinois. The Biopty-Cut needle is manufactured by Bard Urological, Covington Georgia. The Biopty Gun, is manufactured by Bard Radiology, Covington, Georgia. The Promag 2.2 gun is manufactured by Manan Medical Products, Northbrook, Illinois.
History of Medicare Coverage Policies
Although generally covered by local carriers, the advent of percutaneous image-guided breast biopsy has resulted in claims of confusion regarding the appropriate CPT coding. Presently, no national coverage exists. Physicians have reported performance of image guided breast biopsies using a variety of CPT codes, including 19100, 19101, and 19120 (Table 5). Until recently, most Medicare Carriers had allowed payments based only upon CPT code 19100. Medicare currently allows payment for image guided biopsies under CPT code 19101 as well as 19100. Code 19120 is reserved for open surgical biopsy.
Table 5: Relevant CPT Codes
- 19100: Biopsy of breast; needle core
- 19101: Biopsy of breast, Incisional
- 19120: Excision of cyst, fibroadenoma, or other benign or malignant tumor aberrant breast tissue, duct lesion, nipple or areolar lesion, male or female, one or more lesions
- 19499: Unlisted procedure, breast
- 76095: Stereotactic localization for breast biopsy, each lesion, radiological supervision and interpretation
- 76942: Ultrasonic guidance for needle biopsy, radiological supervision and interpretation
Additionally, 19101 was initially valued as an open incisional breast biopsy. The word "incisional" has been subject to different interpretations. Presently, "incisional" can apply to the technique used for both an open surgical biopsy as well as a percutaneous image-guided breast biopsy.
Due to some confusion in the physician community as well as the medical device industry, in Winter of 1996, Biopsys Medical, Inc. requested clarification of the above CPT codes to establish which codes offered the appropriate means of billing for biopsies performed using the Mammotome device. Biopsys sought permission to use the miscellaneous code (19499) and expressed their intention of applying for a unique CPT code, specific for directional vacuum assisted biopsies. The American Society of Breast Physicians petitioned the American Medical Association to issue a new CPT code for such biopsies. In February 1999 the CPT Editorial Panel denied this request.
Supporters of a new code argue that 19101 does not adequately describe the image-guided breast biopsy procedures, since they were designed for open surgical biopsy, a very different technique than image-guidance. In addition, a separate code (s) would recognize the unique elements of image-guidance biopsy, distinguishing it from OSB. Proponents argue that a new code would eliminate confusion for physicians unsure which code to use, as well as provide a mechanism for appropriate tracking. Of note, several hundred physicians have written in response to the proposed changes in the Physician Fee Schedule, requesting a new code for image-guided breast biopsy.
This history and possible confusion concerning breast biopsy coverage and payment, suggests a need for clarification of our coverage policy of breast biopsy. This document focuses on the coverage policy and will not address payment and coding.
Timeline of Recent HCFA Activities
In June, 1999 Biopsys sent HCFA materials which they believed supported a change in Medicare policy regarding percutaneous breast biopsies, with particular emphasis on directional vacuum assisted devices, although not limiting any requested changes solely to their device. Biopsys provided HCFA with materials dated June 7, 1999. This included articles from the medical literature regarding Mammotome and other forms of breast biopsies. These articles are listed in Appendix B and are analyzed in Appendix A [PDF, 370KB]. On June 29, HCFA internalized the issue as a formal request.
HCFA staff met with representatives of Biopsys Medical, Inc on July 23, 1999. At this meeting, clinicians experienced in performing breast biopsies presented information regarding their own experiences and discussed the scientific literature with HCFA staff.
HCFA staff met with representatives of the Mroz-Baier Breast Care Center on August 6, 1999. This meeting involved a discussion of the medical literature as well as the experience with biopsies at the Mroz-Baier Breast Care Center, Memphis, Tennessee.8
On September 20, 1999 HCFA staff met with representatives of US Surgical to exchange scientific information about the use of the ABBI device. These articles are listed in Appendix C and the relevant ones are analyzed in Appendix A [PDF, 370KB].
Throughout the summer and early fall of 1999, HCFA staff reviewed the available medical literature including materials provided by external sources and the results of its own Medline searches. Engaged in extensive dialogue with individual physicians, organized physician groups (including the American College of Radiology) and patients.
Analysis of Scientific Literature
Over 40 peer-reviewed, published scientific articles were evaluated. A detailed analysis of the studies can found in Appendix A [PDF, 370KB].
Percutaneous image-guided breast biopsies are proven safe. HCFA has analyzed numerous published reports and met with several clinicians experienced in performing breast biopsies. The available information suggests that all types of percutaneous image-guided breast biopsies can be performed with a low occurrence of complications. Reported complications of percutaneous image-guided breast biopsies are both infrequent and non-serious. The most commonly reported complications involve infection at the incision site, bleeding, and hematomas. The complication rate for percutaneous biopsies compares favorably to that for open surgical biopsy. Percutaneous biopsies are generally performed more quickly than surgical biopsies and without the need for general anesthesia, decreasing the required recovery time and the risk of the procedure. Estimated complication rates for SCNB are in the range of 0.2%. Estimated complication rates for DVAB procedures are 0.16%
In determining the appropriate indications for percutaneous image-guided breast biopsy, it is necessary to consider the accuracy of such techniques – specifically, the extent to which they over-diagnose and under-diagnose cancer ( e.g. sensitivity, specificity, positive predictive value, negative predictive value)9 These accuracy measures are usually compared to the current generally accepted gold standard for histologic diagnosis, open surgical biopsy. As mentioned earlier, open surgical biopsy is an imperfect gold standard, with an error rate as high as 20% (without wire localization).
Accuracy of percutaneous image-guided biopsies is more difficult to assess than the safety. HCFA has analyzed numerous articles which attempt to study the accuracy of the various procedures. Of particular value, some of the study designs involved patients undergoing a percutaneous biopsy followed by an open surgical biopsy. In some studies follow-up surgical biopsies were performed on all patients, whereas in other studies OSB was performed only for patients with certain initial histologic diagnoses by percutaneous biopsy.
Several studies report that a certain percentage of lesions diagnosed as ADH, LCIS, or DCIS by DVAB or SNCB prove to be cancerous at follow-up open surgical biopsy. This is usually called upgrading of diagnosis and reflects one form of diagnostic inaccuracy. The most recent studies document upgrading less than 10%. Perhaps the most difficult form of diagnostic inaccuracy to detect is the rate of false negative diagnoses. Many of the studies did not provide open surgical biopsies for patients with benign diagnoses by percutaneous image guided biopsy. Most studies rely on mammographic follow-up to detect false negatives, with varying rate in compliance of recommended mammographic follow-up. This makes it difficult to know exactly how many cancerous lesions percutaneous image guided breast biopsy might miss which OSB would have detected. Overall, however, the overwhelming majority of studies reviewed as well as consultation with experts demonstrate that percutaneous image-guidance breast biopsy is as accurate as open surgical biopsy.
There are several advantages of image-guidance breast biopsy that need to be recognized. Obviously, it is much less invasive, and more tolerable by many patients. The procedure typically requires less anesthesia, takes less time, and is performed in a less threatening environment. In addition, an advantage of percutaneous biopsy over surgical biopsy is the reduced chance of the procedure creating artifact to confuse interpretation of future imaging studies. To assist with future follow-up, clinicians often insert a metal marking clip to identify the site of removed tissue. Several studies have also suggested that the directional vacuum-assisted devices and automated surgical biopsy can remove the entire region which produced the mammographic abnormality, especially microcalcifications and lesions less than 5 mm. In the past, such lesions were referred to open surgical biopsy.10 Moreover, if a patient undergoes OSB for initial histology, she has no histologic diagnosis prior to her first surgical procedure. If a patient undergoes percutaneous image-guided breast biopsy initially, she may have a greater opportunity to consider the possible implications of cancer and fully discuss treatment options with her surgeon prior to her first operation. The option of percutaneous image-guided breast biopsy can offer women greater choice.
Given that percutaneous image-guidance breast biopsy is as accurate as open surgical biopsy, which mammographically detected lesions are amenable to diagnosis by percutaneous image-guided biopsy instead of open surgical biopsy? Ideally, the lesion should be nonpalpable. Clearly, if a lesion cannot be palpated, image guidance, either ultrasound or stereotactic guidance, needs to be employed. Which type of image guidance for which type of lesion should be left to the discretion of the treating physician. Some physicians may argue that palpable lesion should not be excluded; however, there is not compelling evidence at this time that image guidance is absolutely necessary for palpable lesions. There may be certain circumstances where image guidance is medically necessary and reasonable for a palpable lesion, and in such cases, image guidance will be covered. Individual contractors should determine with the advice of their Carrier Advisory Committee (CAC) under what circumstances ultrasound and stereotactic guidance will be allowed for palpable lesions. It is important to note that the overwhelming majority of studies submitted and reviewed focused on nonpalpable lesions. In addition, the majority of comments submitted to HCFA from physicians performing image-guidance breast biopsy freely offered as one of their indications for such a technique that the lesion is nonpalpable.11
The BIRADS system offers additional criteria for the appropriateness of image-guidance breast biopsy. For mammographic lesions causing high suspicion of malignancy (i.e. BIRADS V) a percutaneous biopsy yielding a normal pathology report may not fully alleviate the suspicion of cancer and follow-up with open surgical biopsy may often be recommended. The resulting question is what value does an initial percutaneous biopsy serve if both a benign and a malignant pathology report would prompt open surgical biopsy? Some clinicians argue that percutaneous biopsy is not indicated for BIRADS V lesions on the grounds that a test is unnecessary if the result does not tangibly influence patient management in that either result leads to the recommendation of open surgical biopsy. Other clinicians argue that the percutaneous biopsy is useful because, although open biopsy is recommended regardless of the percutaneous histology, the initial biopsy provides useful information which influences the surgical approach, often allowing the tumor to be treated with a single surgical procedure when two might otherwise be required. Supporters claim a presurgical diagnosis of cancer allows discussion of treatment options prior to surgery. Such presurgical diagnosis may also enhance pre-surgical planning to increase the chance of obtaining clear margins with one surgical procedure. Several articles do suggest that obtaining an initial histologic diagnosis by means of percutaneous image guided breast biopsy allows the patient to undergo fewer total surgical procedures for diagnosis and treatment than when the initial histology is obtained by OSB Such planning can be helpful, and patients may prefer such a choice based on an informed decision-making process with her physician.
To address the other end of the spectrum of mammographic suspicion, some clinicians have expressed concern that the availability of percutaneous biopsy might cause an overly high use of this technology to biopsy lesions with a low mammographic suspicion of cancer . The argument follows that many patients and physicians would decline to immediately biopsy lesions which are probably benign (i.e. BIRADS III) if open surgical biopsy were the only biopsy option and would instead elect to closely follow the lesion with repeat examination and mammography. The availability of a less invasive method of biopsy might therefore prompt a drive for histologic diagnosis of lesions which might otherwise be treated more conservatively. However, the availability of less invasive biopsy techniques could also allow more cancers to be detected, and earlier on. Again, patients should have such an option, based on an informed decision-making process with his/her physician. Biopsy of a BIRADS III is a valid choice.
BIRADS IV lesions, suspicious abnormalities, should most certainly be eligible for image-guidance breast biopsy. Some women may not choose image-guidance, but they need to have choice.
The issue of choice is an important consideration. There are few health circumstances that cause a woman distress as the presence of an abnormal mammogram. Given that percutaneous image guidance breast biopsy is as accurate as open surgical biopsy, patients should have a choice as to which technique they wish to undergo.
Although percutaneous image-guided breast biopsy includes DVAB, ASB, and needle core biopsies – all with or without ultrasound /stereotactic guidance except ABBI—DVAB and ASB are distinctly different from needle core biopsy. Therefore, DVAB and ASB should be differentiated from open surgical biopsy as well as needle core biopsy.
DECISION:
Establish a national coverage policy for percutaneous image-guided breast biopsy. Image-guidance include directional, vacuum assisted breast biopsy, automated surgical biopsy, and needle core biopsy. For those lesions that are (1) nonpalpable and (2) BIRADS III, IV, or V, image guidance using stereotactic or ultrasound will be covered. For those lesions that are palpable, individual carriers can decide as to whether or not image guidance will be covered.
1 Ultrasound is generally not an effective tool for routine breast cancer screening because sonographic imaging fails to detect microcalcifications. The main usefulness of ultrasound appears to be enhanced ability to distinguish between solid and cystic masses.
2 This is consistent with the Department of Health and Human Service's efforts to promote greater use of mammography for screening.
3 Hence, the phrase "tissue is the issue"
4 Most experts, however, estimate the error rate to be less than 5%.
5 A table which allows the patient to sit during the procedure also exists, although typically the patient lies prone.
6 There is a recognition that males also are diagnosed with breast cancer; for simplicity, patients are referred to as females in this document.
7 This is in contradistinction to the 5-25 gm removed by OSB, and 100 mg removed by DVAB.
8 Representatives from Mroz-Baier Breast Care Center (Dr. Christine Mroz and Mr. Joseph Baier) also met with representatives from the Center for Health Plans and Providers on May 7.
9 All of these devices are considered diagnostic techniques only.
10 Again, this decision memorandum considers image guidance breast biopsy as diagnostic, not therapeutic techniques.
11 Physicians could still perform percutaneous image guidance on a palpable lesion, but could not bill for the image guidance component unless it was deemed medically necessary and reasonable by the Medicare contractor.


