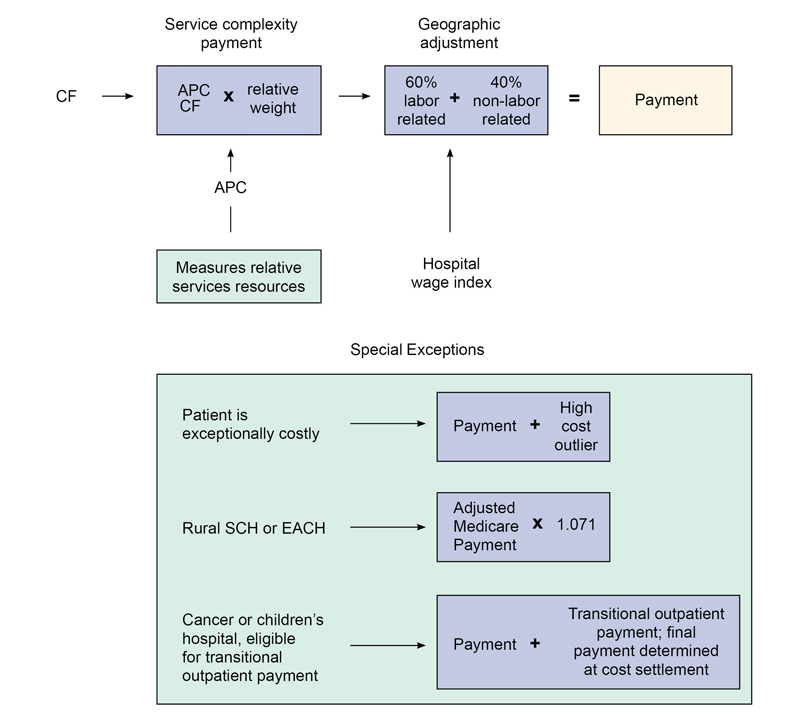
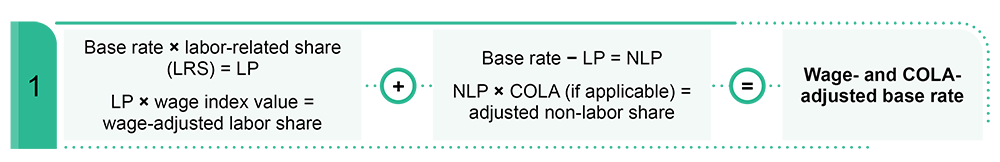
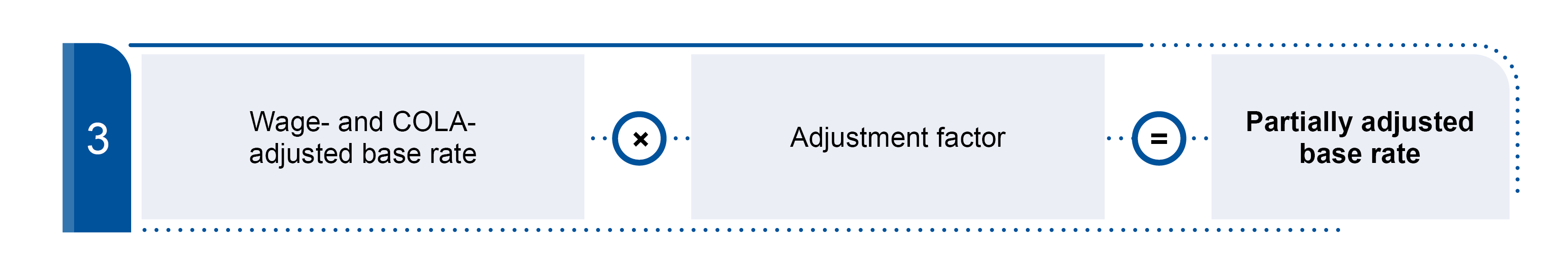
CMS updates the LTCH payment rates annually based on the market basket index. Per federal regulations, we then reduce the rates through a productivity adjustment.
The FY 2026 LTCH Prospective Payment System (PPS) market basket increase factor is 2.7%, which is a 3.4% market basket update reduced by a 0.7 percentage point productivity adjustment.
For LTCHs that don’t report quality data, we further reduce the market basket rate by 2 percentage points.
Medicare Severity Long-Term Care Diagnosis-Related Group Patient Classifications
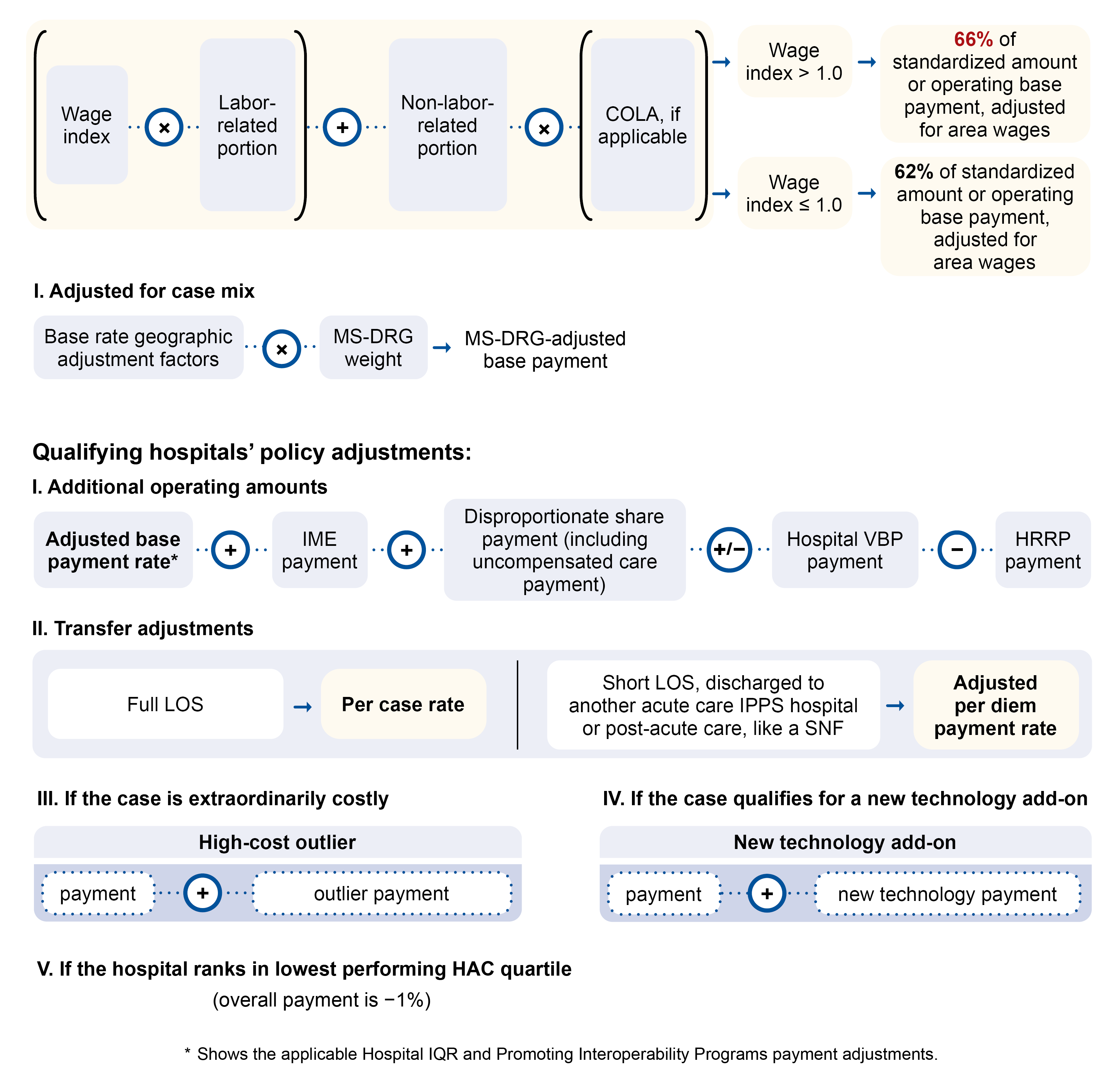
We pay LTCHs under the LTCH PPS. Under this payment system, we set base payment rates for inpatient stays based on:
- Patient’s diagnosis
- Services or treatment provided
- Illness severity
A hospital gets a single payment for each case depending on the payment classification assigned at discharge. The classification systems are:
- Inpatient Prospective Payment System (IPPS): Medicare Severity Diagnosis-Related Groups (MS-DRGs)
- LTCH PPS: Medicare Severity Long-Term Care Diagnosis-Related Groups (MS-LTC-DRGs)
We group each patient stay using:
- One principal diagnosis
- Up to 24 secondary diagnoses
- Up to 25 procedure codes
- Age
- Sex
- Patient discharge status
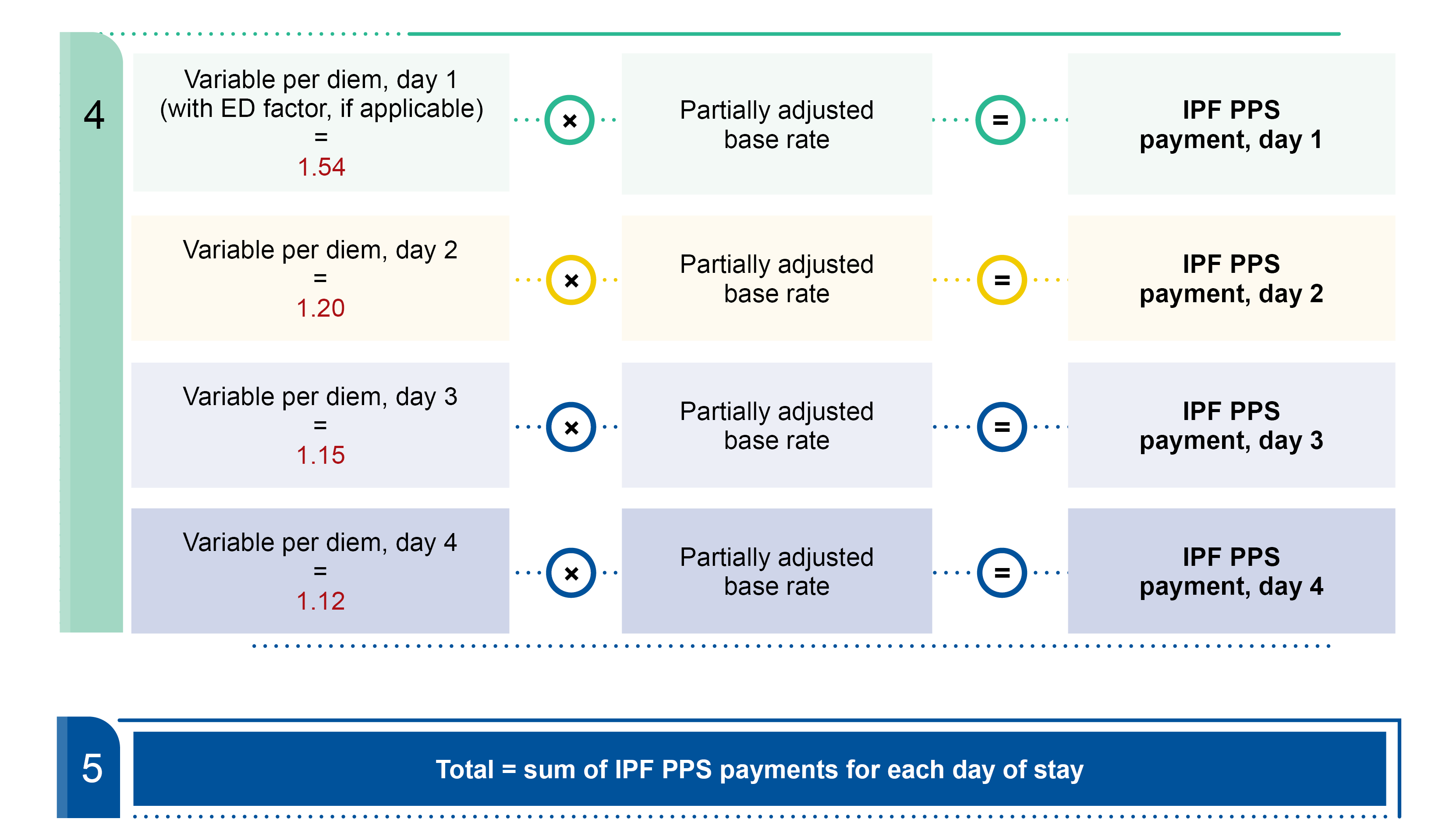
We annually update each MS-LTC-DRG based on the latest LTCH discharge data and its predetermined average length of stay (ALOS). We pay LTCHs for each discharge based on the MS-LTC-DRG if it meets exclusion requirements from the site neutral payment rate. We pay for cases assigned to an MS-LTC-DRG based on the federal payment rate, including payment and policy adjustments.
SSO, HCO, fixed-loss amount, and interrupted stay payment policy adjustments all apply to site neutral and standard federal payment rate discharges except where noted.
Short-Stay Outlier
The SSO policy helps prevent inappropriately paying for cases without a full episode of care. SSO payment adjustments apply only to the standard federal payment rate discharges and may happen when a patient:
- Experiences an acute condition that needs urgent treatment or more intensive rehabilitation and the LTCH discharges them to another facility
- Doesn’t need an LTCH care level and the LTCH discharges them to another facility
- Is discharged to their home
- Dies within the first several days after LTCH admission
- Exhausts LTCH benefits during the stay
We apply an adjustment when the LOS ranges from 1 day through 5/6 of the ALOS for the MS-LTC-DRG where we group that case. We subject the MS-LTC-DRG payment to the SSO adjustment.
We don’t apply an adjustment when the LOS is more than 5/6 of the ALOS for the MS-LTC-DRG where we group that case. In this situation, the LTCH gets the full MS-LTC-DRG payment.
Note: When calculating the SSO adjustment, we cap the SSO threshold (5/6 of the ALOS for the MS-LTC-DRG) at 25 days. We never subject stays of 25 days or more to the SSO policy.
This policy doesn’t apply to site neutral discharges.
We blend the MS-LTC-DRG per diem amount with what we would pay under the IPPS, calculated as a per diem and capped at the full IPPS comparable amount.
SSO Payments When Patient Benefits Exhaust During an LTCH Stay
We base LTCH payments on the patient’s covered benefit days until the LOS triggers a full MS-LTC-DRG payment. This means a patient’s remaining benefit days and length of hospital stay affect LTCH payments and may result in an SSO payment adjustment.
Table 3. Benefits Exhaust & LOS is Below MS-LTC-DRG Threshold
| If |
Then |
Example |
| The patient uses regular episode benefit days during an LOS below the SSO MS-LTC-DRG threshold |
- The patient pays for non-covered days
- The LTCH gets SSO payment for the patient’s covered hospital stay
|
The MS-LTC-DRG SSO threshold is 25 days, and the patient’s LOS is 20 days; the LTCH gets SSO payment. The patient’s benefit days end on day 15.
- We pay for 15 covered days under the LTCH SSO policy
- The patient pays for days 16–20
|
Table 4. Benefits Exhaust & LOS Exceeds MS-LTC-DRG Threshold
| If |
Then |
Example |
| The patient uses all episode benefit days during an LOS exceeding the SSO MS-LTC-DRG threshold |
- The patient doesn’t pay for non-covered days (until they reach HCO threshold)
- The LTCH gets full MS-LTC-DRG payment
|
The MS-LTC-DRG SSO threshold is 25 days, and the patient’s benefit days end on day 30; the patient’s LOS is 35 days.
- The patient doesn’t pay for days 31–35 (the SSO policy doesn’t apply)
- The LTCH gets full MS-LTC-DRG payment; the patient pays for the first day the stay qualifies as an HCO through discharge
|
Note: We allow 90 covered episode benefit days of care under the inpatient hospital benefit. Each patient has 60 lifetime reserve days, which the patient may use to cover non-covered episode days of care exceeding 90 days.
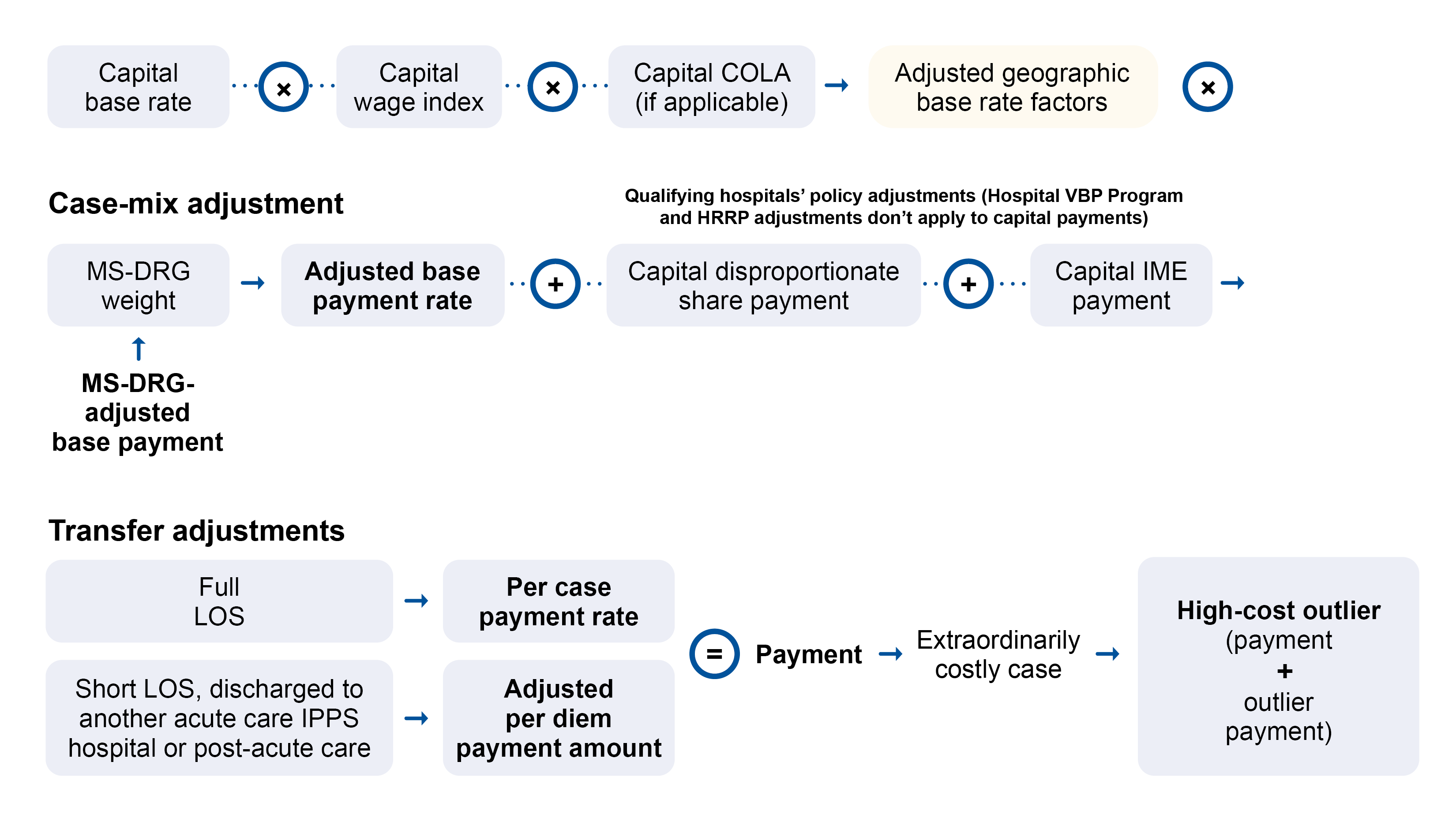
High-Cost Outlier
The HCO policy adjusts the applicable LTCH PPS payment rate (site neutral rate or standard federal rate) for LTCH stays with costs exceeding typical cases of similar case-mix costs. To qualify for an HCO payment, an LTCH’s estimated treatment costs must exceed the outlier threshold. We calculate the applicable outlier threshold as the case’s applicable LTCH PPS payment plus the applicable fixed-loss amount.
The HCO payment equals 80% of the difference between the estimated case cost and the outlier threshold.
For SSO cases, we calculate the outlier threshold by adding the applicable fixed-loss amount to the adjusted SSO MS-LTC-DRG payment. If the estimated SSO case cost exceeds the outlier threshold, it qualifies for an HCO payment.
We set 2 fixed-loss amounts:
- Site neutral payment rate
- Standard federal rate
The HCO adjustment:
- Improves LTCH PPS hospital- and patient-resource cost accuracy
- Cuts LTCH financial losses from treating patients needing more costly care
- Limits LTCH loss to fixed-loss amount and cost percentages above the marginal cost factor
- Discourages underserving high-cost patients
Medicare Administrative Contractors (MACs) use PRICER software to determine if enough medically necessary benefit days are in the outlier period. If a patient has enough benefit days, the MAC processes the claim as usual and the LTCH takes no other action. If a patient doesn’t have enough benefit days, the MAC returns the claim to the LTCH for correction, indicating the correct HCO threshold amount.
HCO Payments When Patients Exhaust Benefits During an LTCH Stay
We make HCO payments for:
- Days the patient has Medicare coverage (regular, coinsurance, or lifetime reserve days) for part of the stay beyond the HCO threshold
- Medically necessary covered cost days when the patient has a benefit day available
Table 5. Patient Benefits Exhaust Before Qualifying for Full LTCH PPS Standard Federal Rate Payment
| If |
Then |
Example |
- The patient’s benefits are exhausted before qualifying for the full MS-LTC-DRG payment
- Covered care costs exceed the standard federal rate HCO threshold for an SSO-adjusted payment
|
The LTCH gets HCO payment with SSO-adjusted payment for covered medically necessary benefit days
|
The LTCH admits a standard federal rate patient with 5 remaining benefit days grouped to an MS-LTC-DRG with a 30-day ALOS.
- The patient doesn’t have enough regular benefit days to trigger a full MS-LTC-DRG standard federal rate payment (5/6 of MS-LTC-DRG ALOS), qualifying the case for an SSO-adjusted payment.
- The LTCH cost for covered services during the 5 benefit days exceeds the standard federal rate HCO threshold. The case also qualifies for an HCO payment for all costs above the HCO threshold days 1–5.
- The patient pays for day 6 through discharge.
|
Table 6. Patient Benefits Exhaust After Qualifying for Full Applicable LTCH PPS Payment
| If |
Then |
Example |
- The patient’s benefits are exhausted after qualifying for the full applicable LTCH PPS payment
- Covered care costs exceed the applicable HCO threshold
|
The LTCH gets HCO payment with the full LTCH PPS payment for covered medically necessary benefit days |
The LTCH admits a standard federal rate patient with 36 remaining benefit days grouped to an MS-LTC-DRG with a 30-day ALOS.
- On day 33, the patient’s care cost exceeds the standard federal rate HCO threshold, qualifying the case for the full MS-LTC-DRG standard federal rate payment and the HCO payment for all covered costs (available benefit days) above the HCO threshold
- The patient pays for day 37 through discharge
|

“Full applicable LTCH PPS payment” means the standard federal rate (including SSO adjustment) or the site neutral payment rate, based on the LTCH case. “Applicable HCO threshold” means the HCO threshold determined from the standard federal rate fixed-loss amount or site neutral fixed-loss amount based on the LTCH case.

Table 7. Patient Exhausts Benefits Before Exceeding Applicable HCO Threshold
| If |
Then |
Example |
- The patient qualifies for the full applicable LTCH PPS payment
- The patient uses all regular benefit days for the stay before exceeding the applicable HCO threshold
|
- The LTCH gets the full LTCH PPS payment (and doesn’t get the HCO payment)
- The patient pays costs incurred the day after the case exceeds the applicable HCO threshold
|
The LTCH admits a standard federal rate patient with 36 remaining benefit days grouped to an MS-LTC-DRG with a 30-day ALOS.
- The patient care cost exceeds the standard federal HCO threshold on day 45
- The patient exhausted all benefit days before reaching the HCO threshold; the case isn’t eligible for HCO payment
- The patient doesn’t pay covered costs for days 37–45
- The patient pays for day 46 through discharge
|
If the patient’s benefits are exhausted during the LTCH stay, determine the:
- Day when the case cost reaches the applicable HCO threshold (use charges per day and CCR)
- Number of benefit days the patient has left
To calculate the HCO, use the costs for the days after the patient’s case cost reaches the HCO threshold of available benefit days. If the patient remains under care after benefits are exhausted, they pay the costs of those remaining days.
Any changes to HCO payments under the LTCH PPS outlier reconciliation policy don’t retroactively affect a patient’s lifetime reserve days or coverage status, benefits, or payments under Medigap or Medicaid.
HCO Fixed-Loss Amounts
The fixed-loss amount for standard federal payment rate cases is the amount allowing yearly projected total HCO payments to equal 7.975% of the total LTCH PPS standard federal payment rate payments estimated for that year (full MS-LTC-DRG payments or adjusted SSO amount plus HCO payments).
We include estimated uncompensated care payments in the outlier fixed-loss cost threshold calculation. Specifically, in all cases, we use the estimated per-discharge uncompensated care payments to hospitals eligible for these payments.
The applicable HCO threshold for site neutral payment rate cases is the sum of the case’s site neutral payment rate and the IPPS fixed-loss amount. We set the site neutral case fixed-loss amount to the same value as the IPPS fixed-loss amount.
We estimate each case’s cost using provider-specific file CCRs.
Use the applicable statewide average CCR when the LTCH’s provider-specific file CCRs aren’t available.
MACs estimate a case’s cost by multiplying the Medicare-covered charges by the LTCH’s overall CCR, based on the most recently settled or tentatively settled cost report.
These CCR revisions or determinations may also apply:
- We may ask MACs to use an alternative CCR showing recent substantial increases or decreases in a hospital’s charges
- LTCHs may ask their MAC to use a higher or lower CCR based on substantial evidence when their CMS Regional Office approves it
- MACs annually assign the statewide average CCR to LTCHs with CCRs above the maximum ceiling
- MACs use an LTCH’s actual CCR rather than the statewide average LTCH CCR with CCRs below the minimum floor
- MACs may use the statewide average CCR when the LTCH CCR is undetermined (for example, before a new LTCH submits its first Medicare cost report or when data isn’t available to calculate the CCR because it’s missing or incorrect)
The LTCH PPS outlier policy allows for reconciling HCO payments at cost report settlement and accounts for differences between the estimated and actual CCR for the period when the discharge occurs.
Interrupted Stay
An interrupted stay happens when an LTCH discharges a patient to an acute care hospital, inpatient rehabilitation facility (IRF), skilled nursing facility (SNF), swing bed, or home, and the same LTCH readmits the patient for more medical treatment within a specified period. For example, an LTCH discharges the patient for treatment because the services are unavailable in the LTCH.
The 2 types of interrupted stays are:
- 3 days or less
- Greater than 3 days
The interruption day count starts the day of discharge (the first day the patient is away from the LTCH at midnight).
3-Day or Less Interruption Example
If an LTCH discharges a patient on September 2, the 3-day or less interrupted stay policy determines payment if the LTCH readmits the patient to the same LTCH on September 2, 3, or 4.
If an LTCH discharges a patient and readmits them to the same LTCH within 3 days, the patient may:
- Get outpatient or inpatient tests, treatment, or care at an inpatient acute care hospital, IRF, SNF, or swing bed. Outpatient or inpatient care during interruption is part of a single LTCH care episode and bundled into the LTCH payment. If a patient gets tests or procedures during a 3-day interruption and the LTCH pays the provider under arrangements, the total patient day count includes all interrupted days.
- Have an intervening patient stay at home for up to 3 days with no tests, treatment, or care. If the patient doesn’t get care during the 3-day interruption, the LTCH can’t use days away in the total LOS. However, if the patient gets care during an interruption the LTCH pays for under arrangements, the LTCH uses all interruption days in that patient’s LOS.
Greater Than 3-Day Interruption Example
If a patient is discharged from an LTCH on September 2, the greater than 3-day interrupted stay policy determines payment if the patient is readmitted to the same LTCH between September 5 and the applicable provider’s fixed-period threshold.
For a greater than 3-day interruption, the LTCH must:
- Discharge the patient
- Admit them directly to an inpatient acute care hospital, IRF, SNF, or swing bed
- Readmit them to the original LTCH within a specified period
Table 8. Greater Than 3-Day Interruption
| Discharge To |
Interrupted Stay Fixed Period |
| Inpatient acute care hospital |
Between 4 and 9 consecutive days |
| IRF |
Between 4 and 27 consecutive days |
| SNF or swing bed |
Between 4 and 45 consecutive days |
We treat an interrupted stay episode as 1 discharge for payment and make 1 LTCH PPS payment. Interrupted stays are eligible for HCO payments.
We pay separately for an intervening inpatient stay at the acute care hospital, IRF, SNF, or swing bed.
These examples don’t qualify as interrupted stays:
- The patient’s stay at an acute care inpatient hospital, IRF, SNF, or swing bed exceeds the fixed-day period
- The patient is discharged to a facility type other than an acute care inpatient hospital, IRF, SNF, or swing bed
- The patient is discharged to more than 1 facility or goes home between LTCH stays
If the stay disruption doesn’t meet the interrupted stay definition, the original discharge ends the patient’s first stay. If an LTCH readmits the patient, the second admission starts a new stay. The LTCH gets 2 LTCH PPS payments (full MS-DRG payment or adjusted SSO payment, as applicable) for 2 patient stays:
- Payment for the first stay
- Payment for the stay after an LTCH readmission
Interrupted Stay Billing Requirements
- Report the stay’s dates in Statement Covers Period:
- The from date is the original admission date
- The through date is the final discharge date
- Report payable days in the Covered Days field (value code 80)
- Report interrupted days in the Non-Covered Days field (value code 81)
- Report occurrence span code (OSC) 74 with the dates the patient is absent at midnight (interruptions of more than 1 day):
- OSC from date is the initial LTCH discharge date
- OSC through date is the last LTCH date the patient isn’t present at midnight
- Don’t change the principal diagnosis when the patient is readmitted to the LTCH; if the patient has other medical conditions when they return, report the diagnosis codes on the claim
- Use revenue code 018X to show the number of interruption days
Discharge Payment Percentage Adjustment
An LTCH’s discharge payment percentage is the ratio of the LTCH’s discharges that got the standard federal rate payment to its total Medicare discharges under the LTCH PPS. If an LTCH’s discharge payment percentage for a cost reporting period isn’t at least 50%, this payment adjustment policy applies after we calculate the percentage and notify the LTCH. For cost reporting periods subject to this adjustment, the discharge payment percentage adjustment is:
- An amount equivalent to the hospital IPPS payment
- An added HCO-cases payment based on the fixed-loss amount for an IPPS hospital in effect at the time of the LTCH discharge
The payment adjustment ends when the calculated cost reporting period’s discharge payment percentage is at least 50%. We may subject the LTCH to this adjustment again if, after reinstatement, the discharge payment percentage falls below 50%.
LTCHs subject to a cost reporting period payment adjustment can get a special probationary reinstatement. They can do this by getting the payment adjustment delayed if, for at least 5 consecutive months of the 6 months before the cost reporting period, they calculate the discharge payment percentage to at least 50%.
For any cost reporting period where the payment adjustment would have applied without a delay, the payment adjustment will be applied for all discharges in the cost reporting period if the discharge payment percentage isn’t at least 50%.