IMPACT Act Standardized Patient Assessment Data Elements
Introduction
The Improving Medicare Post-Acute Care Transformation Act of 2014 (IMPACT Act) requires that standardized patient assessment data elements (SPADEs) be collected across post-acute care (PAC). Standardized data will enable cross-setting data collection, outcome comparison, exchangeability of data, and comparison of quality within and across PAC settings. In addition, standardized data has the potential to improve patient outcomes by improving coordination of care and discharge planning.
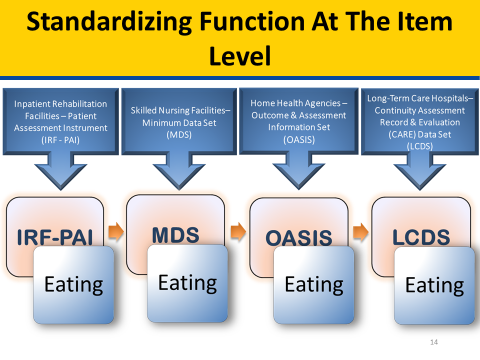
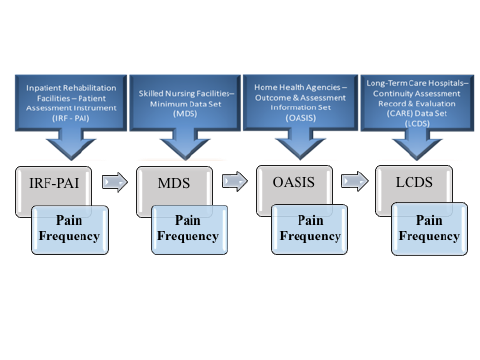
SPADEs are to be nested within the four existing PAC assessment instruments. Existing PAC assessment instruments by PAC provider type are Outcome and Assessment Information Set (OASIS) for HHAs, Inpatient Rehabilitation Facility–Patient Assessment Instrument (IRF-PAI) for IRFs, LTCH Continuity Assessment Record and Evaluation (CARE) Data Set (LCDS) for LTCHs, and Minimum Data Set (MDS) for SNFs. The figure below illustrates how a standardized “Pain Frequency” item could be used across PAC provider types by being included in the patient/resident assessment item set.
Announcements
January 11, 2019
On November 27th, 2018, on behalf of the Centers for Medicare & Medicaid Services (CMS), the RAND Corporation hosted a meeting in which they presented an overview of results from the National Beta Test of candidate standardized patient assessment data elements (SPADEs) and answered questions from attendees. Presentation materials, a transcript of the session, and Frequently Asked Questions related to the presentation are available on CMS' IMPACT Act Downloads and Videos page. CMS invites feedback on this presentation and on the SPADEs tested in the National Beta Test. Please submit input by sending an email to SPADEForum@rand.org. Feedback is requested by February 1, 2019. A verbatim comment summary report will be posted on the CMS website, but CMS will not be responding to comments.
August 15, 2018
CMS is currently in the field testing standardized patient assessment data elements (SPADEs). The field period for the National Beta Test is closing soon. Data analysis of the national field test data, as well as several stakeholder and expert input opportunities taking place in Fall 2018 include: a meeting of the TEP in mid-September, webinars with advocates of special populations in October, and a Forum on Data Element Standardization in November.
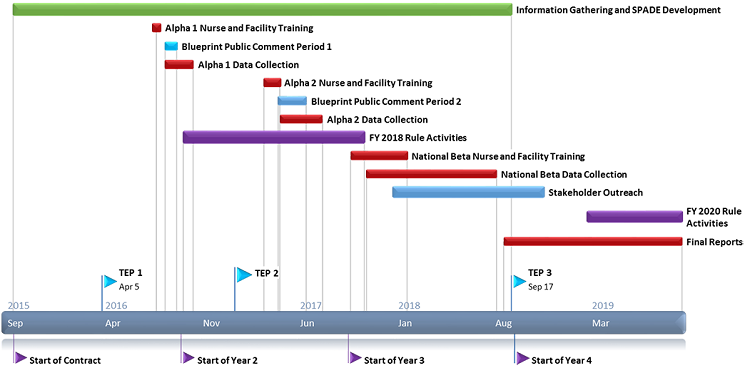
SPADE Development and Testing – Activity Timeline
This effort began in October 2015 and will continue through 2019.
- Information Gathering and SPADE Development: Sep 2015-Sept 2018
- Pilot Testing (Alpha 1 and 2): Aug 2016-July 2017
- National Beta Testing: Nov 2017-Aug 2018
- Data Analysis and Reporting results: Sept 2018-Sept 2019
Information Gathering and SPADE Development Activities
Information gathering activities began at the start of the project in October 2015. In the first year of the project, these activities included systematic literature review; development of conceptual frameworks; consultation with subject matter expert (SME) advisors, focus groups, and a first convening of the Technical Expert Panel (TEP); and a first subregulatory public comment period.
Information gathering continued in the second year of the project and included the Alpha feasibility tests (conducted August to October 2016 and April to July 2017; described below), a second convening of the TEP (January 2017), and a second subregulatory public comment period.
The National Beta test (described below) is ongoing, ending August 2018. The Beta field period will be followed by a third convening of the TEP in September 2018. The FY 2020/CY2021 proposed rule activities will begin in March 2019.
Pilot and National Testing
The overarching goal of the pilot and national tests is to evaluate the reliability and validity of candidate SPADEs and identify the best, most feasible subset for standardization to meet requirements of the IMPACT Act. In each testing phase, assessment data were collected by trained “Research Nurses,” as well as trained facility/agency staff from each participating provider. The role of the Research Nurse is to oversee field data collection in their market and to serve as a “gold standard” coder alongside a facility/agency staff person, allowing measurement of interrater reliability (IRR).
To evaluate empirical evidence as to feasibility/ease of use, we examined the time spent to complete the items and the number of cases in which the research nurse and facility/agency staff left the item missing or indicated “unable to assess/no response.” To determine whether items could be completed with acceptable IRR, we calculated the level of agreement between paired assessors’ coded item responses.
Alpha (Pilot) Testing
The Alpha 1 Test (August to October 2016) was the first phase of pilot testing candidate SPADEs. Testing was conducted among 4 PAC providers (one of each PAC type) in the greater Hartford, Connecticut area. Research Nurses and facility/agency staff conducted 133 paired assessments so that results could be compared for both feasibility and interrater reliability. The Alpha 1 Feasibility Test report is available on the IMPACT Act Downloads page.
The Alpha 2 Test (April to July 2017) was conducted among 15 PAC providers in three regions of the United States (Chicago, Houston, and Denver). As with Alpha 1 testing, Research Nurses and facility/agency staff conducted paired assessments (n=204) so that results could be compared for both feasibility and IRR. The Alpha 2 Feasibility Test report(PDF) (PDF) is available on the IMPACT Act Downloads page.
National Beta Testing
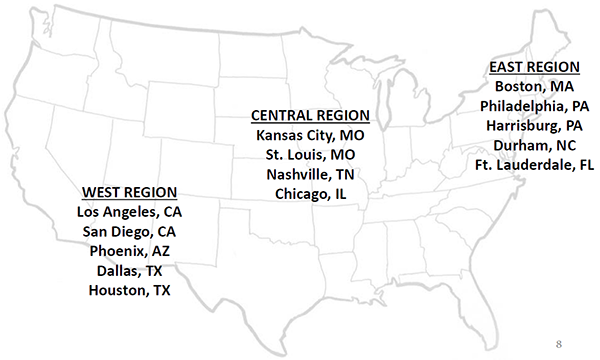
The National Beta test will include 10 months of data collection (ongoing from November 2017 to August 2018) in 142 PAC facilities and agencies across 14 US markets (see map below).
To test performance of candidate SPADEs for different types of patients/residents, as well as the effect of timing of administration on results, we developed three types of protocols:
- Communicative admission assessment, to be administered upon admission to a PAC site among communicative patients/residents who could make themselves understood using any means (i.e., writing, gesturing, speaking);
- Communicative discharge assessment, to be administered at or near discharge from a PAC site among communicative patients/residents who could make themselves understood using any means (i.e., writing, gesturing, speaking);
- Non-communicative assessment, to be administered at any point during a qualifying PAC stay among non-communicative patients/residents who were unable to make themselves understood in any fashion. The candidate SPADEs under evaluation in the National Beta test are as follows:
The protocols for Beta can be located in the downloads section below.
The candidate SPADEs under evaluation in the National Beta test are as follows:
|
Communicative (Admission and Discharge) |
Non-Communicative |
|
Hearing, Vision, Expression, and Understanding Cognition PROMIS Global Health Pain Mood Care Preferences Continence Behavioral Signs and Symptoms Medication Reconciliation Special Services, Treatments, and Interventions |
|
Stakeholder Engagement and Expert Input Activities
Upcoming:
- Targeted webinars for special populations: Fall 2018
- SODF on PAC Data Element Standardization: November 2018
- TEP 3 meeting: September 2018
Previous:
- TEP 1 meeting: April 2016
- Blueprint Public Comment Period 1: August – September 2016
- Public Comment Report published to CMS website: Dec 2017
- SODF: Dec 2017
- TEP 2 meeting: January 2017
- Blueprint Public Comment Period 2: April – June 2017
- Outreach to PAC stakeholders (interviews and conference presentations ): January – June 2018
- SODF: March 2018
- SODF: June 2018
More Information:
- IMPACT Act SPADE FAQs August 2018 – see downloads section below.
- RAND Summary Reports – see IMPACT Act Downloads page.
- CMS IMPACT Mailbox for comments/ideas: PACQualityInitiative@cms.hhs.gov
- IMPACT Item Development: IMPACTbeta-test@rand.org
Downloads
-
IMPACT Act National Testing FAQs- May 2017.pdf (PDF) -
National Field Test Assessment Protocol_Non-Communicative.pdf (PDF) -
National Field Test Assessment Protocol_Admission.pdf (PDF) -
National Field Test Assessment Protocol_Discharge.pdf (PDF) -
IMPACT Act Standardized Assessment National Testing Fact Sheet - May 2017.pdf (PDF)